Marfan syndrome (MFS) is an inherited genetic disorder, primarily affecting the connective tissue that provides strength, support, and elasticity to vital parts of the body (1). The connective tissue is made up of various proteins, including fibrillin-1 (FBN1) (1). FBN1 serves as the main structural component of microfibrils in the extracellular matrix (ECM). These microfibrils incorporate elastin into elastic fibers, thus forming the structural framework. MFS is caused by mutations in the FBN1 gene, which causes misfolding/alteration of FBN1 protein (2, 3).
MFS has a low incidence rate, and it occurs with similar frequency across all countries, races, and genders (3). It is a long-lasting and life-threatening disease affecting multiple vital organs simultaneously, including eyes, the skeletal system, the cardiovascular system, the pulmonary system, the central nervous system, and the integumentary system (4). Currently, a curative therapy for MFS is not available. Patients with MFS often complain of chronic fatigue, pain, and psychological despair (5). MFS patients often require long-term care assistance and have a poor quality of life. The molecular understanding of MFS has advanced greatly in the past 10 years due to the availability of genetically modified rodent models and novel molecular research techniques. Data from MFS patients and animal models show that there is more than one genetic loci linked with MFS, which questions the hypothesis of MFS being a classical dominant negative disorder (6, 7). Primarily, the cardiovascular complications have been attributed to the FBN1 mutations.
Taken together, these findings demonstrate that deregulated TGFβ signaling correlates with the loss of functional microfibrils and may be the chief contributor to the musculoskeletal complications associated with MFS (8). These discoveries regarding MFS pave the way for developing novel pharmacotherapies, which is the standard MFS care prior to surgical intervention. This review will provide a brief overview of the historical advances of MFS, significant clinical manifestations, genetic aspects, current diagnostic criteria, and existing treatment strategies.
Historical outlook: advances in MFS
In 1896, MFS was discovered and named after a French pediatrician, Antoine-Bernard Marfan who found several skeletal disorders in a 5-year-old girl (9). Few decades later, in 1955, McKusick (10) classified MFS as a heritable disorder of the connective tissue, with autosomal dominant pattern. MFS-associated aneurysm and aorta dissection were the main causes of mortality (11). Surgical treatment of MFS patients with aneurysm was considered the only effective treatment option. In 1971, Halpern et al. (12) were the first to find in a small group of patients that β-adrenergic blocker slowed the dilatation of the ascending aorta. This preliminary report later led to a landmark study by Shores et al. (13), with 93 patients' participation that demonstrated the therapeutic benefit of β-blockers. The effectiveness of β-adrenergic blockers in the medical management of cardiovascular complications makes them the chief drug category for MFS treatment to date (13).
In 2001, another breakthrough in pharmacotherapy targeting MFS was made by Nagashima et al. (14), wherein they found a novel regulatory role of angiotensin II type 2 receptor in mediating vascular smooth muscle cell apoptosis in cystic medial degeneration, a complication of MFS. This study led to the evolution of angiotensin-converting enzyme inhibitor (ACE inhibitor) for the treatment of MFS. ACE inhibitors reduce angiotensin II level and its signaling pathway. A randomized trial from 2003 to 2006 with 18 MFS patients enrolled showed that ACE inhibitors effectively reduce aortic root size (15). ACE inhibitors have been used in patients who have unacceptable adverse events or no response to β-blockers. Advancements in the diagnostic criteria of MFS were also occurring simultaneously with the therapeutic progress. Accordingly in 1986, with the ultimate aim to identify MFS patients, the Berlin nosology was introduced, and later in 1996 and 2010, the Ghent-1 and Ghent-2 nosologies, respectively, were introduced with improved diagnostic criteria for MFS (1, 9, 16).
The advances in MFS therapeutic strategies were in line with the discoveries related to the pathophysiology of MFS. In 1991, Dietz and Pyeritz (17) identified and established the genetic mutation link between FBN1 gene and MFS. In 2003, Neptune et al. (18), in a FBN1-deficient animal model, demonstrated that TGFβ activation and signaling were also deregulated. Increased TGFβ signaling is associated with the increased expression of several metalloproteinases (MMPs) and excessive proteolysis of ECM. Subsequently, it was discovered that patients with mutation in the TGFβ receptor type II gene (TGFBR2) showed classic MFS-related phenotypes, which is currently classified as type 2 MFS (MFS2), without significant ocular manifestation (19). This study also mapped TGFBR2 to the MFS2 locus. In 2005, mutations in transforming growth factor-β receptor type I gene (TGFBR1) were found in an MFS-related disorder called Loeys-Dietz aortic aneurysm syndrome (20). These findings bring new insight to the genetic disorders associated with MFS and provide novel therapeutic targets. To date, it appears that all potential genetic loci have not been identified, and several MFS-associated dysfunctions are still unclear with regard to mechanistic aspects. Figure 1 provides a brief outline of the major scientific discoveries associated with MFS.
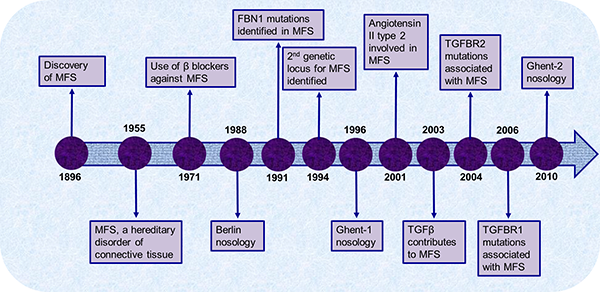
Figure 1. Overview of the major scientific breakthroughs made since the discovery of Marfan syndrome.
Clinical manifestations
MFS affects both the genders equally and has a broad geographical distribution. The syndrome is considered rare, with a detection range of 1.5-17.2 per 100,000 individuals in different populations (16, 21). MFS is often characterized by a plethora of clinical manifestations that classically involves the ocular, cardiovascular, and muscular and skeletal systems. These clinical symptoms, which are mentioned below, become more evident with the increasing age.
Ocular system
Myopia (nearsightedness) and ectopia lentis (malposition of eye lens) are the most common symptoms of MFS that affect the ocular system (40 and 60% of the patients) (1, 9). Other possible symptoms include retinal detachment, glaucoma, and early cataract development. Retinal detachment is considered the most severe ocular complication, often affecting both eyes. However, novel techniques in the clinics have facilitated early diagnosis of the ocular complications, leading to an improved care of MFS patients (22, 23).
Musculoskeletal system
Individuals having MFS also exhibit most striking symptoms involving the skeletal and connective tissue systems. These mainly include ligamentous laxity (loose joints), dolichostenomelia (abnormal lengthening of the limbs), pectus excavatum (sunken chest) or pectus carinatum (protruded chest), and scoliosis (deformed spine) (24). Some of the other significant clinical MFS symptoms found include craniofacial deformities, dural ectasia (erosion of bony tissue), protrusio acetabuli (displacement of the acetabulum and femoral head), hindfoot valgus with forefoot abduction (misalignment of the hindfoot), pes planus (flat foot), and osteopenia (reduced bone mineral density) (25-27). Skeletal malformations, which severely affect the fitness of MFS patients, are often accompanied with extreme pain.
Cardiovascular system
Typically, abnormalities of the cardiovascular system represent the lethal manifestations of MFS. Some of the important and common presentations include aortic root dilatation and mitral valve prolapse (28, 29). Aortic root dilatation was observed in 60% of a series of patients with MFS (74% males, 33% females) while mitral valve prolapse was predominant in 91% (87% males, 100% females) of patients (29). Interestingly, a study detected aortic root dilatation and mitral valve prolapse in all the 13 cases of MFS (30). Children often manifest other heart defects, such as coarctation (narrowing) of the aorta, atrial septal defect, patent ductus arteriosus (persistent opening between two major blood vessels), and pulmonary artery stenosis (narrowing) (31). The aorta and the aortic root are prone to develop dilatation, aneurysm, and dissection. Moreover, the mitral valve tends to develop annular dilatation, fibromyxomatous alterations to the leaflets and chordae, lengthening and rupturing of chordae, and accumulation of calcium (32, 33).
Other systems
Clinical reports have also demonstrated abnormalities in the respiratory and central nervous systems and in the skin (4, 34-37). In the central nervous system, the MFS patients exhibit lumbosacral dural ectasia as a common manifestation (4). Particularly, spontaneous pneumothorax (air or gas in between the lungs and the chest wall), apical blebs, and bullous emphysema (overinflation of the air sacs) are respiratory system abnormalities associated with MFS (38, 39). The rupturing of the apical blebs leads to the frequent spontaneous pneumothorax in MFS patients (39). MFS patients also often exhibit typical stretch marks (striae), skeletal muscle hypoplasia, and adipose tissue deficiency.
Diagnostic criteria
MFS is a pleiotropic disease with a wide range of manifestations in different organ systems. Owing to the multiorgan nature of the disease, the diagnosis of MFS becomes very challenging. Several nosologies have been postulated to standardize the diagnostic criteria and thus clinically define the disease for early diagnosis. With the ultimate aim to identify MFS patients, the Berlin nosology was introduced as the first concerted effort to address the disparity in diagnoses (16). The Berlin nosology relied wholly on the clinical features that did not allow successful delineation of MFS from other syndromes that affected the ocular and skeletal systems. Although the Berlin nosology had limited benefit for diagnosis, several shortcomings were identified. Of greatest concern were the misdiagnoses of patients by relying solely on this nosology (40). Many more weaknesses surfaced over the years, and with the advancement of molecular research, a revised Ghent nosology was introduced in 1996 (Ghent-1). Notably, Ghent-1 was the outcome of the discovery of FBN1 gene mutations as the etiology of MFS (1, 9).
More recently, in an attempt to reduce the number of false-positive diagnoses using Ghent-1 nosology, a revised version was introduced in 2010 (Ghent-2) (41). Readers are directed to the article by von Kodolitsch et al. (16) for a detailed comparison of diagnostic criteria for MFS according to the Berlin and Ghent-1/2 nosologies. The urgent need for a more reliable and uniform diagnostic criteria was the primary reason behind the formulation of the Ghent-2 nosology. It primarily encompasses the following revisions: identification of patients at risk for aortic aneurysm or dissection and ectopia lentis; ease of use of diagnostic criteria; availability of early diagnosis through genetic testing at reasonable costs; enhanced characterization of familial ectopia lentis, the MASS (mitral valve prolapse, aortic enlargement, skin and skeletal findings) syndrome, and mitral valve prolapse syndrome (MVPS); complete removal of some clinical criteria atypical of majority of MFS patients; and, finally, delineation of MFS from alternative diagnoses of other syndromes, such as Loeys-Dietz syndrome and Shprintzen-Goldberg syndrome (16, 41-43). Aortic dilatation, one of the most important diagnostic criteria, is defined by a z-score because z-score refers exclusively to the aortic root (44). Broadly, the occurrence of ectopia lentis together with an FBN1 mutation along with aortic dilatation is sufficient to make the MFS diagnosis.
Evaluation using the Ghent-2 nosology diminishes the inaccuracies in the diagnoses of MFS when compared with other nosologies (45, 46). However, the method to predict z-score can be challenging with both pediatric and adult patients, and it warrants more review. Also, taking into consideration the additional complex criteria such as age and gender may exacerbate this issue. In summary, the quality of diagnostic criteria of a specific nosology comprises objectivity, reliability, and validity, which have not been thoroughly assessed in both Ghent-1 and Ghent-2 that may have led to their incapability to diagnose MFS with 100% accuracy (Table 1). Most notably, a nosology-based diagnosis of MFS lacks a diagnostic reference standard, and hence, its sensitivity, specificity, or accuracy cannot be measured. Nosologies may evolve in future to overcome these diagnostic limitations and provide a more conclusive diagnosis of MFS.
Table 1. Summary of diagnostic yield in suspected MFS patients according to Ghent-1 versus Ghent-2 nosology among different studies
|
Study |
Diagnosis of MFS by Ghent-1 |
Diagnosis of MFS by Ghent-2 |
Indefinite diagnosis of MFS by Ghent-1 |
Indefinite diagnosis of MFS by Ghent-2 |
|---|---|---|---|---|
|
Aalberts et al. (46) |
44/343 (13%) |
47/343 (14%) |
203/343 (59%) |
178/343 (52%) |
|
Sheikhzadeh et al. (47) |
126/300 (42%) |
128/300 (43%) |
84/300 (28%) |
92/300 (31%) |
|
Yang et al. (45) |
86/106 (81%) |
84/106 (79%) |
14/106 (14%) |
14/106 (15%) |
|
All studies combined |
256/749 (34%) |
259/749 (35%) |
301/749 (40%) |
284/749 (38%) |
Molecular genetics: origins of MFS
Fibrillin-1
The biological mechanisms driving the initiation and progression of MFS are yet to be entirely elucidated. MFS is an inherent autosomal dominant disorder in which FBN1 mutations exert a dominant negative impact (32). Fibrillin-1 is an integral constituent of the microfibrils of the ECM in the connective tissues (48). It is a large glycoprotein of about 350 kDa, mainly comprising 6-cysteine epidermal growth factor (EGF)-like and calcium-binding EGF motifs, intermixed with a few 8-cysteine motifs (TGF-beta binding/8-Cys) (Figure 2). This arrangement of the motifs is exclusive to fibrillins and latent TGFβ-binding proteins (48, 49). The FBN1 gene comprises 65 coding exons and resides on chromosome 15q21.1 (Figure 2). Missense mutations, alternative splicing, multiexon out-of-frame deletions, and deletions in this gene result in defective protein folding, altered secretion/assembly, and upregulated degradation of the nonfunctional protein, leading to weakened connective tissues (26, 50, 51). Alternatively, whole gene deletions have also been reported, leading to reduced amounts of protein. Irrespective of the type of mutation and the subsequent altered or deleted functional protein, it has been observed that the overall levels of the immunoreactive fibrillin-1 protein are strikingly low in the affected tissues in MFS patients (52). This strongly indicates that the loss of intact fibrillin-1 protein contributing to functional microfibrils in the ECM corresponds to the MFS clinical phenotype (53). According to the latest update from the UMD-FBN1 mutation database, more than thousand (1847) different mutations have been identified in association with MFS, but not all have the reliability to predict the exact clinical phenotype (54) (http://www.umd.be/FBN1/). Solitary exception is the mutations within the middle third (exon 24-32) of FBN1 gene observed in children and adults with severe MFS (30, 55). Interestingly, only 28-66% of MFS patients have been diagnosed with FBN1 mutations (33, 54, 56). These mutations are not only limited to MFS but also occur in familial aortic aneurysms, MVPS (57, 58), and a wide range of skeletal (e.g., scoliosis) (59), dermal (e.g., stiff skin) (60), and connective tissue abnormalities (fibrillinopathies) (61)
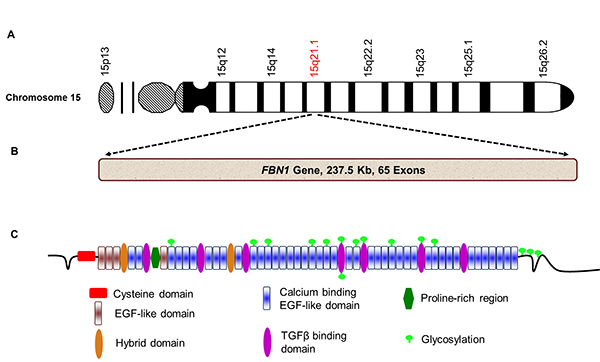
Figure 2. Schematic illustration of fibrillin-1 gene, its location on chromosome 15, and fibrillin-1 protein. A. FBN1 gene is located on chromosome 15, specifically in the 15q21.1 region. B. FBN1 gene comprises 65 exons and is 237.5 kb in length. C. FBN1 translates into a large glycoprotein with multiple functional domains called fibrillin-1 (2871 amino acids, 350 kDa). Glycosylation is a common post-translational modification in this protein that is critically involved in ECM stability.
Apart from MFS, there are rare instances in which FBN1 mutations can also drive other clinically distinctive syndromes. Some of the syndromes include Weill-Marchesani syndrome, stiff skin syndrome, acromelic dysplasia, and Shprintzen-Goldberg syndrome (42, 62, 63). This hampers the establishment of a concrete and prognostic genotype-phenotype correlation among patients with different FBN1 mutations (50). The commonalities and distinct clinical features between MFS and these syndromes are beyond the scope of this review. However, readers are directed to the Online Mendelian Inheritance in Man (OMIM) compendium for further information (http://www.ncbi.nlm.nih.gov/omim).
TGFβ signaling genes
In the early 1990s, a second genetic locus (3p24.2-p25) was identified as a putative cause of MFS (64). In patients with overlapping clinical features of Loeys-Dietz syndrome and MFS, mutations were detected in the transforming growth factor-β receptor type II gene (TGFBR2) (19, 65). Incidentally, TGFBR2 maps to the same new locus identified as an alternative cause of MFS. Subsequently, a plethora of studies was established, investigating the role of TGFβ signaling in the pathogenesis of MFS (18, 20, 66). Using functional approaches, mutations were also detected in another receptor for TGFβ, the TGFBR1 (20). Thus, the significance of TGFβ signaling pathway was confirmed in MFS and similar clinical phenotypes.
During tissue repair or remodeling, TGFβ signaling regulates ECM formation by binding of the TGFβ ligand to its receptors (TGFBR1/2/3) (67, 68). The failure of repair/remodeling processes was accompanied by alarmingly high plasma levels of TGFβ ligand. With the association of TGFBR1/2 being established with MFS, subsequent research was conducted to investigate the role of TGFBR3 genetic variation in MFS and related syndromes. TGFBR3 is the most abundantly expressed TGFβ receptor subtype and possesses high affinities toward all the TGFβ isoforms; recently, its association with MFS was investigated (69). Remarkably, in a cohort of 49 patients, fulfilling the diagnostic criteria for MFS and negative for mutation in FBN1, TGFBR1, or TGFBR2 coding regions, screening for mutations in TGFBR3 gene revealed no exonic or intronic TGFBR3 variation causing the disease (69). However, the role of TGFBR3 variants as a genetic modifier cannot be neglected and needs further investigation.
Furthermore, Smad2 plays an essential role in the TGFβ canonical signaling pathway contributing to the pathogenesis of MFS (Figure 3) (70, 71). By forming a complex with Smad4 and other proteins, Smad2 regulates transcription and induces MMP production (72). MMPs can then cleave the microfibrillar network, other components of the ECM, and latency-associated protein (LAP). Finally, in vivo experiments in mice have demonstrated the importance of targeting the TGFβ noncanonical signaling pathway as a means to alleviate clinical symptoms of MFS. Erk1/2 and p38-MAPK inhibition led to reduced MMP levels that play a pivotal role in progressive remodeling of arterial wall in MFS (Figure 3) (73-75). However, due to the conflicting data on involvement of these pathways in MFS, it is yet to be fully understood which pathway is more influential in the pathogenesis of MFS.
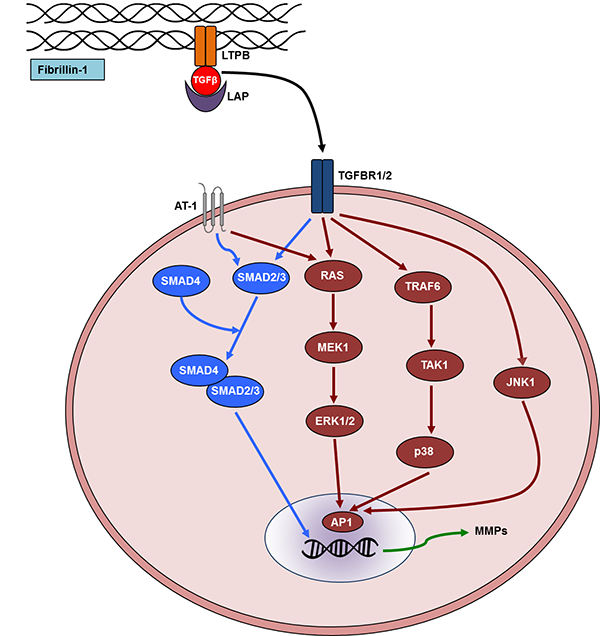
Figure 3. Schematic representation of transforming growth factor-beta (TGF-β) signaling pathway. Fibrillin-1 is an integral constituent of the microfibrils of the extracellular matrix in the connective tissue. TGFβ is secreted in the ECM containing latency-associated protein (LAP). This complex binds to the TGFβ-binding protein (LTPB), which adheres to the microfibrils. When the activated TGFβ ligand binds to one of its receptors (TGFBR1/2), it activates canonical (blue) or noncanonical (brown) signaling pathway in the cytoplasm. Particularly, SMAD-mediated canonical signaling and noncanonical signaling that involves p38, extracellular signal-regulated kinase (ERK1/2), and Jun N-terminal associated kinase (JNK1) are shown. Matrix metalloproteinases are directly upregulated by TGFβ signaling that further exacerbates the clinical phenotype in MFS by promoting aneurysms. The AT-1 receptor of angiotensin II is also depicted in the diagram that is a current therapeutic target. MMP, matrix metalloproteinases; TGFBR1-2, TGFβ-binding receptor 1-2; RAS, rat sarcoma; TAK1, TGF-β-activated kinase 1; TRAF6, TNF receptor-associated factor; MEK1, mitogen-activated protein kinase 1; AP-1, activator protein 1 is a transcription factor in the nucleus.
Management: diagnosis and treatment
MFS is a multiorgan disorder, primarily affecting the ocular, cardiovascular, and connective tissues in the body. Because of its wide range of clinical manifestations, management of this disease becomes challenging. An integrated care system comprising experts from all the relevant fields can effectively manage this deadly disease (76). Cardiovascular complications being the most life threatening, several researchers have focused on strategies to specifically alleviate cardiovascular abnormalities. Once diagnosed with cardiovascular abnormalities, echocardiographic imaging of the aorta at regular intervals is a crucial procedure to monitor the rate of aneurysm progression, aortic root dilatation, and other cardiac anomalies (77, 78). Generally, blood pressure control and limited physical activities are often suggested to MFS patients, depending on the severity of the disease (79, 80). Improvements in mitral valve prolapse, aortic dilatation, and aortic dissection can drastically increase the quality of life and life expectancy. Following are the current treatment strategies for MFS.
β-blockers
β-blockers
are the drugs that bind to
β-adrenoceptors, widely used against numerous cardiovascular complications.
The use of prophylactic β-blockers (e.g., propranolol and atenolol) has
been the preferred treatment option to relax the dilatation of aorta (77).
These drugs have the capacity to lower the mean slope of regression line
for the aortic root dimensions as compared to controls (11, 18). Subsequent
follow-up results showing diminished progressive dilatation of the aorta have
further confirmed the therapeutic benefit of β-blockers. Although
β-blockers are the standard care for MFS, there are insufficient data demonstrating
its therapeutic potential, especially in children (81). However,
β-blockers in combination with calcium antagonist therapy have shown a
significant inhibition of aortic growth rate in children and adults with MFS
(82).
Angiotensin II receptor blockers and ACE inhibitors
Advancements in molecular research have revealed that aberrant TGFβ signaling and altered fibrillin-1 protein have a causative role in the pathogenesis of MFS. Hence, it was proposed that the use of TGFβ antagonists, including angiotensin II receptor blockers, might considerably alleviate some of the clinical manifestations of MFS, such as aortic root dilatation and aneurysm (1, 15). Administration of valsartan, an angiotensin II receptor blocker, and perindopril, an ACE inhibitor, has demonstrated significant reduction in TGFβ signaling (83, 84). Particularly, perindopril therapy effectively reduced arterial stiffness and aortic root diameters in patients with MFS (84). Furthermore, in a study comparing a β-blocker (atenolol) and an ACE inhibitor (losartan) in children with rapidly progressing cardiovascular disorder, losartan demonstrated a significant reduction in the rate of aortic dilatation versus the group treated with β-blocker only (77).
MMP blockers
Great deal of research has been undertaken to study MMP inhibitors due to the critical role of MMPs (MMP2/9) in the progression of aneurysms and related cardiovascular complications (85). Additionally, the role of TGFβ signaling in MFS and MMP activation led to significant advances in the development of MMP inhibitors. The efficacy of doxycycline versus atenolol was compared to treat aortic aneurysms in an MFS animal model (75). Specifically, doxycycline exhibited better therapeutic benefit by preserving the integrity of elastic fibers and reducing TGFβ activation. A clinical trial using doxycycline was initiated in 60 patients scheduled for elective open aneurysmal repair to demonstrate its effect on vascular inflammation (86). Future studies could possibly focus more on other MMP inhibitors and also combination therapies to treat MFS effectively.
Surgical interventions
Limited success of pharmacotherapies led to the evolution of newer surgical techniques for MFS care. Composite valve graft replacement is currently the leading treatment option for a wide range of aortic lesions that have high risk of aortic rupture (87, 88). Alternatively, the diameter of the aorta can also be reduced by a procedure called aortoplasty. Reduction aortoplasty has shown promising results in children with MFS who presented dilatation of the proximal ascending aorta and aortic annulus, and aortic insufficiency (89). In adult patients with MFS, Bentall procedure is still an obvious treatment choice (90). First described in 1968, Bentall and De Bono (91) procedure mainly involves composite graft replacement of the aortic valve, aortic root, and ascending aorta. The coronary arteries are then reimplanted into the composite graft, allowing to effectively treat aortic lesions associated with MFS. This cardiac surgery procedure has exhibited significant clinical benefit, low operative mortality, and long-term survival (survival rate of 80% after 5 years and 60% after 10 years). Recently, Yacoub or David II procedure has been introduced, where the Dacron graft is remodeled to reproduce the aortic sinuses. Patient's aortic valve is reimplanted into the graft (David I procedure), minimizing the necessity for anticoagulant therapy and the use of antibiotics (53, 55). Overall, surgical interventions, such as aortic root replacement, mitral valve procedure, and valve-sparing procedures although invasive, have significantly low operative and postoperative mortality rates and significantly high survival rates (92-94).
Conclusions and future considerations
MFS is an uncommon hereditary disorder of the connective tissues, affecting several organ systems simultaneously. Current nosology-based diagnoses incorporate comprehensive assessment of several major and minor clinical manifestations. Nosologies not only incorporating the relevant clinical manifestations but also thoroughly considering other factors, such as age and sex, may evolve in the future to overcome the limitations of Ghent-2 nosology, thus providing a more reliable diagnosis of MFS. Although the origins of MFS have been attributed to mutations in the FBN1 gene or altered TGFβ signaling, elucidation of complete pathogenesis of MFS remains an area of future investigation. Complications of the cardiovascular system being the foremost life-threatening symptom, majority of research has focused on developing newer pharmacotherapies and improving surgical interventions. However, it is critical to evaluate the risks and benefits of contemporary and budding medications and surgery techniques. Cutting-edge molecular research techniques and computational biology may aid to identify novel molecular therapeutic targets, which can then be subsequently validated in animal models and clinical trials. Furthermore, delineating the pathophysiological mechanisms of MFS from other similar syndromes using these pioneering research techniques warrants further attention. Currently, genetic screening and cardiac imaging for diagnoses are complex due to the overlapping clinical symptoms of other FBN1-related disorders. Future work should emphasize more on targeted treatment, enhanced disease prediction (genetic aspects, prognosis biomarkers, newer imaging modalities, etc.), and perhaps prevention of MFS.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Acknowledgment
We thank members of the Leong-Poi lab and our collaborators at St. Michael's hospital for their valuable contributions to some of the results discussed in this review.
References
1. Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005; 366(9501): 1965-76. http://dx.doi.org/10.1016/S0140-6736(05)67789-6
2. Stahl-Hallengren C, Ukkonen T, Kainulainen K, Kristofersson U, Saxne T, Tornqvist K, et al. An extra cysteine in one of the non-calcium-binding epidermal growth factor-like motifs of the FBN1 polypeptide is connected to a novel variant of Marfan syndrome. J Clin Invest 1994; 94(2): 709-13. http://dx.doi.org/10.1172/JCI117389
3. Kainulainen K, Karttunen L, Puhakka L, Sakai L, Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet 1994; 6(1): 64-9. http://dx.doi.org/10.1038/ng0194-64
4. Pyeritz RE. The Marfan syndrome. Ann Rev Med 2000; 51: 481-510. http://dx.doi.org/10.1146/annurev.med.51.1.481
5. Bathen T, Velvin G, Rand-Hendriksen S, Robinson HS. Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors. Am J Med Genet Part A 2014; 164A(8): 1931-9. http://dx.doi.org/10.1002/ajmg.a.36574
6. Lucarini L, Evangelisti L, Attanasio M, Lapini I, Chiarini F, Porciani MC, et al. May TGFBR1 act also as low penetrance allele in Marfan syndrome? Int J Cardiol 2009; 131(2): 281-4. http://dx.doi.org/10.1016/j.ijcard.2007.07.048
7. Attias D, Stheneur C, Roy C, Collod-Beroud G, Detaint D, Faivre L, et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation 2009; 120(25): 2541-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.887042
8. Benke K, Agg B, Szilveszter B, Tarr F, Nagy ZB, Polos M, et al. The role of transforming growth factor-beta in Marfan syndrome. Cardiol J 2013; 20(3): 227-34. http://dx.doi.org/10.5603/CJ.2013.0066
9. De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996; 62(4): 417-26. http://dx.doi.org/10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R
10. McKusick VA. The cardiovascular aspects of Marfan's syndrome: a heritable disorder of connective tissue. Circulation 1955; 11(3): 321-42.
11. Jortner R, Shahin W, Eshkol D, Gueron M, Levy MJ. Cardiovascular manifestations and surgery for Marfan's syndrome. Chest 1969; 56(1): 24-30.
12. Halpern BL, Char F, Murdoch JL, Horton WB, McKusick VA. A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment. Johns Hopkins Med J 1971; 129(3): 123-9.
13. Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med 1994; 330(19): 1335-41. http://dx.doi.org/10.1056/NEJM199405123301902
14. Nagashima H, Sakomura Y, Aoka Y, Uto K, Kameyama Ki, Ogawa M, et al. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan's syndrome. Circulation 2001; 104(12 Suppl 1): I282-7.
15. Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC, 3rd. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med 2008; 358(26): 2787-95. http://dx.doi.org/10.1056/NEJMoa0706585
16. von Kodolitsch Y, De Backer J, Schuler H, Bannas P, Behzadi C, Bernhardt AM, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet 2015; 8: 137-55. http://dx.doi.org/10.2147/TACG.S60472
17. Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet 1995; 4 Spec No: 1799-809.
18. Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003; 33(3): 407-11. http://dx.doi.org/10.1038/ng1116
19. Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 2004; 36(8): 855-60. http://dx.doi.org/10.1038/ng1392
20. Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005; 37(3): 275-81. http://dx.doi.org/10.1038/ng1511
21. Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr Opin Genet Dev 2007; 17(3): 252-8. http://dx.doi.org/10.1016/j.gde.2007.04.006
22. Nemet AY, Assia EI, Apple DJ, Barequet IS. Current concepts of ocular manifestations in Marfan syndrome. Surv Ophthalmol 2006; 51(6): 561-75. http://dx.doi.org/10.1016/j.survophthal.2006.08.008
23. Nahum Y, Spierer A. Ocular features of Marfan syndrome: diagnosis and management. Israel Med Assoc J 2008; 10(3): 179-81.
24. Tsipouras P, Silverman DI. The genetic basis of aortic disease. Marfan syndrome and beyond. Cardiol Clin 1999; 17(4): 683-96.
25. Jones KB, Sponseller PD, Erkula G, Sakai L, Ramirez F, Dietz HC, 3rd, et al. Symposium on the musculoskeletal aspects of Marfan syndrome: meeting report and state of the science. J Orthopaed Res 2007; 25(3): 413-22. http://dx.doi.org/10.1002/jor.20314
26. Canadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol 2010; 7(5): 256-65. http://dx.doi.org/10.1038/nrcardio.2010.30
27. Ahn NU, Sponseller PD, Ahn UM, Nallamshetty L, Rose PS, Buchowski JM, et al. Dural ectasia in the Marfan syndrome: MR and CT findings and criteria. Genet Med 2000; 2(3): 173-9. http://dx.doi.org/10.109700125817-200005000-00003
28. Morse RP, Rockenmacher S, Pyeritz RE, Sanders SP, Bieber FR, Lin A, et al. Diagnosis and management of infantile Marfan syndrome. Pediatrics 1990; 86(6): 888-95.
29. Brown OR, DeMots H, Kloster FE, Roberts A, Menashe VD, Beals RK. Aortic root dilatation and mitral valve prolapse in Marfan's syndrome: an echocardiographic study. Circulation 1975; 52(4): 651-7.
30. Sisk HE, Zahka KG, Pyeritz RE. The Marfan syndrome in early childhood: analysis of 15 patients diagnosed at less than 4 years of age. Am J Cardiol 1983; 52(3): 353-8.
31. Papaioannou AC, Agustsson MH, Gasul BM. Early manifestations of the cardiovascular disorders in Marfan syndrome. Pediatrics 1961; 27: 255-68.
32. Erentug V, Polat A, Kirali K, Akinci E, Yakut C. Cardiovascular manifestations and treatment in Marfan syndrome. Anadolu kardiyoloji dergisi (Anatol J Cardiol) 2005; 5(1): 46-52.
33. Yuan SM, Jing H. Marfan's syndrome: an overview. Sao Paulo Med J 2010; 128(6): 360-6.
34. Dwyer EM, Jr., Troncale F. Spontaneous pneumothorax and pulmonary disease in the Marfan syndrome. Report of two cases and review of the literature. Ann Intern Med 1965; 62: 1285-92.
35. Hall JR, Pyeritz RE, Dudgeon DL, Haller JA, Jr. Pneumothorax in the Marfan syndrome: prevalence and therapy. Ann Thorac Surg 1984; 37(6): 500-4.
36. Tanoue LT. Pulmonary involvement in collagen vascular disease: a review of the pulmonary manifestations of the Marfan syndrome, ankylosing spondylitis, Sjogren's syndrome, and relapsing polychondritis. J Thorac Imag 1992; 7(2): 62-77.
37. Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax 1984; 39(10): 780-4.
38. Corsico AG, Grosso A, Tripon B, Albicini F, Gini E, Mazzetta A, et al. Pulmonary involvement in patients with Marfan Syndrome. Panminerva Med 2014; 56(2): 177-82.
39. Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration. Int Rev Thorac Dis 2011; 82(3): 219-24. http://dx.doi.org/10.1159/000322958
40. Rose PS, Levy HP, Ahn NU, Sponseller PD, Magyari T, Davis J, et al. A comparison of the Berlin and Ghent nosologies and the influence of dural ectasia in the diagnosis of Marfan syndrome. Genet Med 2000; 2(5): 278-82. http://dx.doi.org/10.109700125817-200009000-00002
41. Faivre L, Collod-Beroud G, Ades L, Arbustini E, Child A, Callewaert BL, et al. The new Ghent criteria for Marfan syndrome: what do they change? Clin Genet 2012; 81(5): 433-42. http://dx.doi.org/10.1111/j.1399-0004.2011.01703.x
42. Sood S, Eldadah ZA, Krause WL, McIntosh I, Dietz HC. Mutation in fibrillin-1 and the Marfanoidcraniosynostosis (Shprintzen-Goldberg) syndrome. Nat Genet 1996; 12(2): 209-11. http://dx.doi.org/10.1038/ng0296-209
43. Ades LC, Holman KJ, Brett MS, Edwards MJ, Bennetts B. Ectopia lentis phenotypes and the FBN1 gene. Am J Med Genet Part A 2004; 126A(3): 284-9. http://dx.doi.org/10.1002/ajmg.a.20605
44. Radonic T, de Witte P, Groenink M, de Bruin-Bon RA, Timmermans J, Scholte AJ, et al. Critical appraisal of the revised Ghent criteria for diagnosis of Marfan syndrome. Clin Genet 2011; 80(4): 346-53. http://dx.doi.org/10.1111/j.1399-0004.2011.01646.x
45. Yang JH, Han H, Jang SY, Moon JR, Sung K, Chung TY, et al. A comparison of the Ghent and revised Ghent nosologies for the diagnosis of Marfan syndrome in an adult Korean population. Am J Med Genet Part A 2012; 158A(5): 989-95. http://dx.doi.org/10.1002/ajmg.a.34392
46. Aalberts JJ, Thio CH, Schuurman AG, van Langen IM, van der Pol BA, van Tintelen JP, et al. Diagnostic yield in adults screened at the Marfan outpatient clinic using the 1996 and 2010 Ghent nosologies. Am J Med Genet Part A 2012; 158A(5): 982-8. http://dx.doi.org/10.1002/ajmg.a.35343
47. Sheikhzadeh S, Kade C, Keyser B, Stuhrmann M, Arslan-Kirchner M, Rybczynski M, et al. Analysis of phenotype and genotype information for the diagnosis of Marfan syndrome. Clin Genet 2012; 82(3): 240-7. http://dx.doi.org/10.1111/j.1399-0004.2011.01771.x
48. Robinson PN, Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet 2000; 37(1): 9-25.
49. Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res 2010; 339(1): 71-82. http://dx.doi.org/10.1007/s00441-009-0822-x
50. Waldmuller S, Muller M, Warnecke H, Rees W, Schols W, Walterbusch G, et al. Genetic testing in patients with aortic aneurysms/dissections: a novel genotype/phenotype correlation? Eur J Cardiothorac Surg 2007; 31(6): 970-5. http://dx.doi.org/10.1016/j.ejcts.2007.02.027
51. Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E, et al. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet 2007; 81(3): 454-66. http://dx.doi.org/10.1086/520125
52. Hollister DW, Godfrey M, Sakai LY, Pyeritz RE. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med 1990; 323(3): 152-9. http://dx.doi.org/10.1056/NEJM199007193230303
53. Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGF beta and BMP signaling. Curr Opin Cell Biol 2009; 21(5): 616-22. http://dx.doi.org/10.1016/j.ceb.2009.05.005
54. Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med 2001; 161(20): 2447-54.
55. Tiecke F, Katzke S, Booms P, Robinson PN, Neumann L, Godfrey M, et al. Classic, atypically severe and neonatal Marfan syndrome: twelve mutations and genotype-phenotype correlations in FBN1 exons 24-40. Eur J Hum Genet 2001; 9(1): 13-21. http://dx.doi.org/10.1038/sj.ejhg.5200582
56. Robinson PN, Booms P, Katzke S, Ladewig M, Neumann L, Palz M, et al. Mutations of FBN1 and genotype-phenotype correlations in Marfan syndrome and related 13 fibrillinopathies. Hum Mutat 2002; 20(3): 153-61. http://dx.doi.org/10.1002/humu.10113
57. Wang WJ, Han P, Zheng J, Hu FY, Zhu Y, Xie JS, et al. Exon 47 skipping of fibrillin-1 leads preferentially to cardiovascular defects in patients with thoracic aortic aneurysms and dissections. J Mol Med 2013; 91(1): 37-47. http://dx.doi.org/10.1007/s00109-012-0931-y
58. Pepe G, Nistri S, Giusti B, Sticchi E, Attanasio M, Porciani C, et al. Identification of fibrillin 1 gene mutations in patients with bicuspid aortic valve (BAV) without Marfan syndrome. BMC Med Genet 2014; 15: 23. http://dx.doi.org/10.1186/1471-2350-15-23
59. Buchan JG, Alvarado DM, Haller GE, Cruchaga C, Harms MB, Zhang T, et al. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum Mol Genet 2014; 23(19): 5271-82. http://dx.doi.org/10.1093/hmg/ddu224
60. Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med 2010; 2(23): 23ra20. http://dx.doi.org/10.1126/scitranslmed.3000488
61. Faivre L, Collod-Beroud G, Callewaert B, Child A, Loeys BL, Binquet C, et al. Pathogenic FBN1 mutations in 146 adults not meeting clinical diagnostic criteria for Marfan syndrome: further delineation of type 1 fibrillinopathies and focus on patients with an isolated major criterion. Am J Med Genet Part A 2009; 149A(5): 854-60. http://dx.doi.org/10.1002/ajmg.a.32809
62. Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, et al. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet 2011; 89(1): 7-14. http://dx.doi.org/10.1016/j.ajhg.2011.05.012
63. De Backer J, Loeys B, Leroy B, Coucke P, Dietz H, De Paepe A. Utility of molecular analyses in the exploration of extreme intrafamilial variability in the Marfan syndrome. Clin Genet 2007; 72(3): 188-98. http://dx.doi.org/10.1111/j.1399-0004.2007.00845.x
64. Collod G, Babron MC, Jondeau G, Coulon M, Weissenbach J, Dubourg O, et al. A second locus for Marfan syndrome maps to chromosome 3p24.2-p25. Nat Genet 1994; 8(3): 264-8. http://dx.doi.org/10.1038/ng1194-264
65. Singh KK, Rommel K, Mishra A, Karck M, Haverich A, Schmidtke J, et al. TGFBR1 and TGFBR2 mutations in patients with features of Marfan syndrome and Loeys-Dietz syndrome. Hum Mutat 2006; 27(8): 770-7. http://dx.doi.org/10.1002/humu.20354
66. Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8): 788-98. http://dx.doi.org/10.1056/NEJMoa055695
67. Pepin MC, Beauchemin M, Collins C, Plamondon J, O'Connor-McCourt MD. Mutagenesis analysis of the membrane-proximal ligand binding site of the TGF-beta receptor type III extracellular domain. FEBS Lett 1995; 377(3): 368-72. http://dx.doi.org/10.1016/0014-5793(95)01378-4
68. Cheifetz S, Bassols A, Stanley K, Ohta M, Greenberger J, Massague J. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J Biol Chem 1988; 263(22): 10783-9.
69. Singh KK, Schmidtke J, Keyser B, Arslan-Kirchner M. TGFBR3 variation is not a common cause of Marfan-like syndrome and Loeys-Dietz-like syndrome. J Negat Results Biomed 2012; 11: 9. http://dx.doi.org/10.1186/1477-5751-11-9
70. Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFbeta1. J Cell Biol 2007; 176(3): 355-67. http://dx.doi.org/10.1083/jcb.200608167
71. Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, et al. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J Biol Chem 2009; 284(9): 5630-6. http://dx.doi.org/10.1074/jbc.M806962200
72. Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGFbeta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer 2012; 12: 26. http://dx.doi.org/10.1186/1471-2407-12-26
73. Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res 2012; 110(12): e92-101. http://dx.doi.org/10.1161/CIRCRESAHA.112.268268
74. Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011; 332(6027): 358-61. http://dx.doi.org/10.1126/science.1192149
75. Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 2008; 102(8): e73-85. http://dx.doi.org/10.1161/CIRCRESAHA.108.174367
76. Dietz HC. Marfan syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al, editors. Gene Reviews(R). Seattle, WA: University of Washington, Seattle; 1993. PMID:20301510.
77. Tahernia AC. Cardiovascular anomalies in Marfan's syndrome: the role of echocardiography and beta-blockers. Southern Med J 1993; 86(3): 305-10.
78. Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation 2005; 111(11): e150-7. http://dx.doi.org/10.1161/01.CIR.0000155243.70456.F4
79. Maron BJ, Chaitman BR, Ackerman MJ, Bayes de Luna A, Corrado D, Crosson JE, et al. Working Groups of the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004; 109(22): 2807-16. http://dx.doi.org/10.1161/01.CIR.0000128363.85581.E1
80. Iams HD. Diagnosis and management of Marfan syndrome. Curr Sports Med Rep 2010; 9(2): 93-8. http://dx.doi.org/10.1249/JSR.0b013e3181d4066c
81. Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr 2007; 150(1): 77-82. http://dx.doi.org/10.1016/j.jpeds.2006.09.003
82. Rossi-Foulkes R, Roman MJ, Rosen SE, Kramer-Fox R, Ehlers KH, O'Loughlin JE, et al. Phenotypic features and impact of beta blocker or calcium antagonist therapy on aortic lumen size in the Marfan syndrome. Am J Cardiol 1999; 83(9): 1364-8.
83. Xu W, Song S, Huang Y, Gong Z. Effects of perindopril and valsartan on expression of transforming growth factor-beta-Smads in experimental hepatic fibrosis in rats. J Gastroenterol Hepatol 2006; 21(8): 1250-6. http://dx.doi.org/10.1111/j.1440-1746.2006.04331.x
84. Ahimastos AA, Aggarwal A, D'Orsa KM,
Formosa MF, White AJ, Savarirayan R, et al. Effect of perindopril on large
artery stiffness and aortic root diameter in
patients with Marfan syndrome: a
randomized controlled trial. JAMA 2007; 298(13): 1539-47. http://dx.doi.org/10.1001/jama.298.13.1539
85. Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 2008; 47(1): 166-72; discussion 172. http://dx.doi.org/10.1016/j.jvs.2007.09.016
86. Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 2009; 119(16): 2209-16. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.806505
87. Pacini D, Ranocchi F, Angeli E, Settepani F, Pagliaro M, Martin-Suarez S, et al. Aortic root replacement with composite valve graft. Ann Thorac Surg 2003; 76(1): 90-8.
88. Karck M, Kallenbach K, Hagl C, Rhein C, Leyh R, Haverich A. Aortic root surgery in Marfan syndrome: comparison of aortic valve-sparing reimplantation versus composite grafting. J Thorac Cardiovasc Surg 2004; 127(2): 391-8. http://dx.doi.org/10.1016/j.jtcvs.2003.07.049
89. Rammurthy DV, Iyer PU, Kumar A, Mohan M, Khandpur SC. Marfan's syndrome in a neonate. Indian Pediatr 1986; 23(11): 956-9.
90. Patel ND, Weiss ES, Alejo DE, Nwakanma LU, Williams JA, Dietz HC, et al. Aortic root operations for Marfan syndrome: a comparison of the Bentall and valve sparing procedures. Ann Thorac Surg 2008; 85(6): 2003-10; discussion 2010-01. http://dx.doi.org/10.1016/j.athoracsur.2008.01.032
91. Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968; 23(4): 338-9.
92. Gillinov AM, Zehr KJ, Redmond JM, Gott VL, Deitz HC, Reitz BA, et al. Cardiac operations in children with Marfan's syndrome: indications and results. Ann Thorac Surg 1997; 64(4): 1140-4; discussion 1144-5.
93. de Oliveira NC, David TE, Ivanov J, Armstrong S, Eriksson MJ, Rakowski H, et al. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2003; 125(4): 789-96. http://dx.doi.org/10.1067/mtc.2003.57
94. Alexiou C, Langley SM, Charlesworth P, Haw MP, Livesey SA, Monro JL. Aortic root replacement in patients with Marfan's syndrome: the Southampton experience. Ann Thorac Surg 2001; 72(5): 1502-7; discussion 1508.