Key points
• N-acetylcysteine (NAC) has been reported to reduce symptoms of influenza.
• Administration of NAC is thought to have contributed to the successful treatment of patients infected with H1N1.
• Conversely, some studies have shown a null effect of NAC on certain strains of influenza virus.
• The effect of NAC on influenza A virus is strain dependent.
• Susceptibility of influenza viruses to NAC cannot be considered as universal.
• Anti-influenza property of NAC should be interpreted in terms of strains.
Introduction
N-acetylcysteine (NAC), the acetylated variant of the amino acid L-cysteine (molecular formula: HSCH2CH(NHCOCH3)CO2H; Figure 1A), has been used for decades as an adjuvant therapy for several respiratory conditions, thanks to its mucolytic properties (1). NAC also has anti-oxidant and pro-glutathione (pro-GSH) activities that support its therapeutic use in patients with oxidative stress (2). Its main clinical applications include the treatment of acetaminophen (paracetamol) overdose, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, contrast-induced nephropathy and influenza pneumonia (3, 4). NAC was further described as a chelating agent, notably of methylmercury, a glutamate precursor and a methyl donor allowing the conversion of homocysteine to methionine (5–7). As a consequence, NAC administration has been suggested in the management of methylmercury intoxication, cardiovascular diseases and psychiatric conditions (5–8).
Although NAC has been considered as extremely safe, administration of acetylcysteine has been associated with several adverse reactions (9). The most significant adverse reaction of NAC resembles anaphylaxis and has been classified as anaphylactoid reaction. Less common reactions that were reported consisted of electrocardiogram abnormalities, status epilepticus and a syndrome similar to serum sickness (9).
A series of studies addressed the question of the effects of NAC on the outcome of influenza pneumonia (10–22). NAC was shown to have direct anti-viral effects, to decrease the levels of pro-inflammatory cytokines and to be anti-apoptotic (11, 13–17, 19–22). Inhibition of mucin production and effects on B- and T-cell immune responses have also been reported (10, 11, 19). However, although a potent effect of NAC has been described in several models of influenza infection, its efficacy appears as limited for some strains of the virus (12). A better knowledge of the strain-specific pathogenic mechanisms helps to understand these discrepancies.
NAC: a mucolytic agent used since the early 1960s
The first clinical application of NAC was related to its mucolytic properties (1). NAC is a thiol compound, with a free sulfhydryl group (Figure 1A). Thanks to its sulfhydryl moiety; NAC is able to break down disulfide bonds in mucus glycoproteins, which accounts for its “mucolytic” capacities (23, 24) (Figure 1B). These reactions of thiol-disulfide interchange may take place spontaneously or be catalyzed by thiol transferases (25, 26). Mostly used as an adjuvant therapy in patients with cystic fibrosis and chronic obstructive pulmonary diseases (24, 27), NAC has also been suggested as a supportive treatment for influenza pneumonia (11). Recent evidence suggests that a large part of the positive impact of NAC in the treatment of these conditions is actually related to direct and indirect anti-oxidant effects (23).
Anti-oxidant and immunomodulatory activities
A large proportion of clinical signs and lesions associated with respiratory conditions as diverse as asthma, cystic fibrosis or viral pneumonia is related to the inflammatory reaction (23). In particular, inflammation is critical in the pathogenesis of lower respiratory tract viral infections (28, 29). The main effectors of the tissue damage associated with influenza infection are cytokines (the so-called cytokinic storm) and reactive oxygen species (ROS), mostly associated with neutrophils (28). NAC has emerged as a potent and safe anti-oxidant and immunomodulatory drug in the case of influenza pneumonia (11, 13–20, 22).
The anti-oxidant effect of NAC is thought to be dual: direct and indirect (23). NAC, as a thiol compound, is a reducing agent and thus endowed with anti-oxidant properties. However, this direct effect is believed to be negligible at classical in vivo concentrations when compared to its indirect, pro-GSH activity (23). GSH (γ-glutamyl-cysteinyl-glycine) is a highly efficient intracellular tripeptide anti-oxidant, which limits the impact of oxidative stress on lipids, proteins and nucleic acids. NAC, after deacetylation, provides reduced L-cysteine, which is required for the synthesis of GSH (23) (Figure 2). The immunomodulatory properties of NAC, observed both in vitro and in vivo, are thought to be related to the inhibition of the activation of oxidant-sensitive pathways such as those involving nuclear factor (NF)-κB and mitogen-activated protein kinase p38 (14, 19). Levels of cytokines/chemokines after influenza infection were significantly reduced by NAC treatment in vivo (14, 19, 22). NAC treatment also decreased the macrophages, lymphocytes and neutrophils infiltration and myeloperoxidase activity in infected lungs (14, 22). Furthermore, NAC was shown to increase the proliferation of influenza-specific lymphocytes and the effector function of cytotoxic lymphocytes (10).

Figure 1. Structural formula of N-acetylcysteine (NAC; A). Disulfide bonds in proteins (P) can be disrupted by NAC in a two-step thiol-disulfide interchange reaction (B). Adapted from Samuni et al (26).
The interplay between ROS and the NF-κB pathway is complex (30). ROS can modulate the activation of the NF-κB pathway by acting on several factors, located in the cell cytoplasm or in the nucleus, resulting in an either activation or inhibition of the pathway (30). Although out of the scope of the present review, it is worth mentioning here that most of the pro-NF-κB activities of ROS are thought to be related to effects on cytoplasmic factors, mainly the activation of IκB (inhibitor of κB) kinases or alternative phosphorylation of IκBα (30). This is the most likely mechanism by which NAC reduces the activation of NF-κB pathway.
Anti-viral properties and variations between viral strains
Several studies addressed the question about the anti-viral activity of NAC against influenza A virus (Table 1). A dose-dependent inhibition of highly pathogenic H5N1 influenza virus in vitro growth by NAC was demonstrated using two different strains of the virus: A/Thailand/1(Kan-1)/04 and A/Vietnam/1203/04 (14). The effect was maximal (48.9-fold reduction) at 24 hours post infection, with a 15 mM NAC concentration. For the A/Port Chalmers/1/72 (H3N2) influenza strain, NAC treatment (10 mM) reduced the viral titers measured in vitro at 48 hours post infection about 8-fold when compared with untreated controls (19).
In similar conditions, the A/swine/Iowa/4/1976 (H1N1) strain appeared as more resistant to the inhibition by NAC, with a ~5.6-fold reduction of the viral titers at the highest NAC concentration (2.5 mg/ml or 15.3 mM) used by the authors (12). Moreover, with the same viral strain, administration of NAC at a high dosage (100 mg/kg per day) failed to protect mice after viral challenge (12). In similar experiments using the A/Puerto Rico/8/1934 (H1N1) strain, NAC alone only provided a very limited level of protection, but was much more efficient when combined with ribavirin or oseltamivir (13, 15). The in vivo protective effect of NAC was more significant in a mouse model infected by the A/swine/HeBei/012/2008 (H9N2), but unfortunately the effect on viral growth in vitro has not been measured (22).
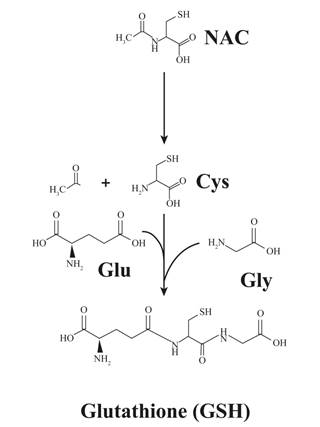
Figure 2. N-acetylcysteine (NAC) as a cysteine donor for glutathione synthesis. Most of the anti-oxidant activity of NAC is thought to be related to its pro-glutathione (GSH) properties. NAC serves as a donor of cysteine residues that are essential to GSH synthesis. Adapted Rushworth and Megson (23).
Table 1. Effect of N-acetylcysteine treatment on viral growth and protection of infected mice for several influenza A strains
|
Influenza A strain |
Inhibition of viral growth |
Level of protection |
References |
|
A/Vietnam/1203/04 et A/Thailand/1(Kan-1)/04 (H5N1) |
++ |
ND |
(14) |
|
A/HongKong/8/68 (H3N2) |
− |
Not significant |
(16) |
|
A/Port Chalmers/1/72 (H3N2) |
+ |
ND |
(19) |
|
A/swine/Iowa/4/1976 (H1N1) |
+ |
− |
(12) |
|
A/Puerto Rico/8/1934 (H1N1) |
ND |
Very limited |
(13, 15) |
|
A/swine/HeBei/012/2008 (H9N2) |
ND |
+ |
(22) |
ND: not determined.
Overall, the inhibition of viral growth and the in vivo protective effects of NAC appear as more pronounced for highly pathogenic strains (12, 14). The lesions associated with the infection of mammals by a highly pathogenic versus a low pathogenic influenza A virus, even with a similar lethality, are extremely different (31). In both cases, diffuse alveolar damage is observed, but the typical target in the pathogenesis of highly pathogenic strains is the endothelium, leading to massive pulmonary edema and hemorrhages. In comparison, infections by low pathogenic strains are associated with a dense leukocytic infiltration of the lung parenchyma, which compromises gas exchanges, but with minimal damage to the alveolar epithelium (31). The endotheliotropism of highly pathogenic strains is believed to be linked to a differential affinity of the viral hemagglutinin for endothelial cells in vitro (32, 33). But it appears that the activation of endothelial cells, more than their infection, is critical in the pathogenesis of H5N1 highly pathogenic influenza (33). Furthermore, the activation of endothelial cells is central in the development of the so-called cytokinic storm, typical of pathogenic influenza strains (34). In particular, a strong activation of the NF-ΚB pathway has been described as a critical step for the expression of genes typically overexpressed after H5N1 influenza infection, including cytokines and chemokines, with a much weaker activation in the case of low pathogenic strains (35). Moreover, pharmacological inhibition of the NF-ΚB pathway proved to be an efficient method to protect against lethal influenza challenges (14, 36). As a consequence, most of the anti-viral activity of NAC against influenza A virus is likely associated with its anti-NF-ΚB properties (14), together with the inhibition of the MAPK p38 pathway. The differences in terms of NF-ΚB activation between highly pathogenic and low pathogenic influenza strains (35) might be the explanation for the variable effects of NAC treatment on viral growth and host resistance between strains.
Through the inhibition of NF-ΚB activation, NAC likely has an additional, indirect effect on influenza growth via a downregulation of α2,6-linked sialic acid receptors expression on target cells, as demonstrated for the closely related drug carbocistein (37). Interestingly, in the same study, carbocistein did not affect the expression of α2,3-linked sialic acid receptors, which raises questions about the underlying mechanism (37). The potential impact of this observation on the variations in susceptibility between influenza strains and NAC treatment is unclear because highly pathogenic avian viruses, such as H5N1 strains, mostly target α2,3-linked sialic acid receptors, whose expression appears as unaffected by carbocistein and, supposedly, NAC (37).
Current clinical use of NAC in influenza patients and perspectives
High dose of NAC has been suggested and used since several years as an adjuvant treatment for influenza pneumonia in human patients (4, 17, 18, 20). NAC was shown to relieve influenza symptomatology and to improve cell-mediated immunity (11). This protective activity was demonstrated for human H1N1 influenza strains, but it is likely that, as detailed above for mouse models, the therapeutic efficacy of NAC is strain dependent. Additional research is clearly needed to determine which human influenza strains are most responsive to NAC treatment to apply this medication only when relevant. Besides, even if NAC is considered a very safe drug, some severe adverse reactions have been reported (9), which raise the question of the risks of using high dose of the drug in patients infected by unresponsive influenza strains. Finally, because most of the anti-influenza activity of NAC is thought to be linked to its anti-NF-ΚB properties (14), it would be interesting to compare the clinical efficacy of NAC with that of more specific anti-NF-ΚB compounds.
Conclusions
NAC has been shown to be an interesting adjuvant therapy in the management of influenza patients, both through experimental infections of mouse models and through clinical evidence from the field. This is reinforced by the in vitro anti-influenza effect of the drug reported by several studies. In spite of this, the level of clinical protection of NAC treatment alone appeared as weak or null in some models with a variation in its efficacy depending on the infecting viral strain. These differences might be related to the anti-NF-ΚB properties of NAC and the variations in terms of NF-ΚB activation between influenza strains. Additional effects of NAC, notably on the expression of some cellular receptors to the virus, might also be involved. Additional research is clearly needed to fully understand the mechanisms underlying the anti-viral effects of NAC and closely related molecules.
References
1. Suddarth SB. Acetylcysteine, a new and effective mucolytic agent. Bull Geisinger. 1963 May;15:65–9.
2. Dekhuijzen PNR. Antioxidant properties of N-acetylcysteine: Their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004 Apr;23(4):629–36.
3. Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane database Syst Rev. 2006; 2:CD003328. http://dx.doi.org/10.1002/14651858.CD003328.pub2.
4. Millea PJ. N-acetylcysteine: Multiple clinical applications. Am Fam Physician. 2009 Aug;80(3):265–9.
5. Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008 Jan;116:26–31. http://dx.doi.org/10.1289/ehp.10383.
6. Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005 Aug;162:1403–1413. http://dx.doi.org/10.1176/appi.ajp.162.8.1403.
7. Dittmann S, Seemüller F, Schwarz MJ, Kleindienst N, Stampfer R, Zach J, et al. Association of cognitive deficits with elevated homocysteine levels in euthymic bipolar patients and its impact on psychosocial functioning: preliminary results. Bipolar Disord. 2007;9:63–70. http://dx.doi.org/10.1111/j.1399-5618.2007.00412.x.
8. Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–82. http://dx.doi.org/10.1007/BF03195624.
9. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila). 2009 Feb;47:81–8. http://dx.doi.org/10.1080/15563650802665587.
10. Boon ACM, Vos AP, Graus YMF, Rimmelzwaan GF, Osterhaus ADME. In vitro effect of bioactive compounds on influenza virus specific B- and T-cell responses. Scand J Immunol. 2002 Jan;55(1):24–32.
11. De Flora S, Grassi C, Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997 Jul;10(7):1535–41.
12. Garigliany M-MO, Desmecht DJ. N-acetylcysteine lacks universal inhibitory activity against influenza A viruses. J Negat Results Biomed. ; 2011;10:5. http://dx.doi.org/10.1186/1477-5751-10-5.
13. Garozzo A, Tempera G, Ungheri D, Timpanaro R, Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int J Immunopathol Pharmacol. 2007;20(2): 349–54.
14. Geiler J, Michaelis M, Naczk P, Leutz A, Langer K, Doerr H-W, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010 Feb;79(3): 413–20. http://dx.doi.org/10.1016/j.bcp.2009.08.025.
15. Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol. 2004;17:99–102.
16. Gowdy KM, Krantz QT, King C, Boykin E, Jaspers I, Linak WP, et al. Role of oxidative stress on diesel-enhanced influenza infection in mice. Part Fibre Toxicol. 2010;7:34. http://dx.doi.org/10.1186/1743-8977-7-34.
17. Hui DSC, Lee N. Adjunctive therapies and immunomodulating agents for severe influenza. Influenza Other Respi Viruses. 2013 Nov;7(Suppl 3):52–9. http://dx.doi.org/10.1111/irv.12171.
18. Lai KY, Ng WY, Osburga Chan PK, Wong KF, Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann Intern Med. 2010;152:687–8. http://dx.doi.org/10.7326/0003-4819-152-10-201005180-00017.
19. Mata M, Morcillo E, Gimeno C, Cortijo J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem Pharmacol. 2011 Sep;82(5):548–55. http://dx.doi.org/10.1016/j.bcp.2011.05.014.
20. McCarty MF, Barroso-Aranda J, Contreras F. Practical strategies for targeting NF-kappaB and NADPH oxidase may improve survival during lethal influenza epidemics. Med Hypotheses. 2010 Jan;74(1):18–20. http://dx.doi.org/10.1016/j.mehy.2009.04.052.
21. Wu H, Song W, Gao X, Liu N, Wang P, Chen H, et al. Proteomics study of N-acetylcysteine response in H1N1-infected cells by using mass spectrometry. Rapid Commun Mass Spectrom. 2014 Apr;28(7):741–9. http://dx.doi.org/10.1002/rcm.6840.
22. Zhang R-H, Li C-H, Wang C-L, Xu M-J, Xu T, Wei D, et al. N-acetyl-l-cystine (NAC) protects against H9N2 swine influenza virus-induced acute lung injury. Int Immunopharmacol. 2014 Sep;22(1):1–8. http://dx.doi.org/10.1016/j.intimp.2014.06.013.
23. Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014 Feb;141(2):150–9. http://dx.doi.org/10.1016/j.pharmthera.2013.09.006.
24. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis. 1964 Nov;90:721–9.
25. Freedman RB. How many distinct enzymes are responsible for the several cellular processes involving thiol:protein-disulphide interchange? FEBS Lett. 1979 Jan;97(2):201–10.
26. Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013 Aug;1830(8):4117–29. http://dx.doi.org/10.1016/j.bbagen.2013.04.016.
27. Tattersall AB, Bridgman KM, Huitson A. Acetylcysteine (Fabrol) in chronic bronchitis--a study in general practice. J Int Med Res. 1983;11(5):279–84.
28. Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri P, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. Canada; 2013 Feb;17(2):e76–83. http://dx.doi.org/10.1016/j.ijid.2012.06.006.
29. Schwarze J, Mackenzie KJ. Novel insights into immune and inflammatory responses to respiratory viruses. Thorax. 2013 Jan;68(1):108–10. http://dx.doi.org/10.1136/thoraxjnl-2012-202291.
30. Morgan MJ, Liu Z. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011 Jan;21(1):103–15. http://dx.doi.org/10.1038/cr.2010.178.
31. Garigliany MM, Habyarimana A, Lambrecht B, Van de Paar E, Cornet A, van den Berg T, et al. Influenza A strain-dependent pathogenesis in fatal H1N1 and H5N1 subtype infections of mice. Emerg Infect Dis. 2010 Apr;16(4):595–603. http://dx.doi.org/10.3201/eid1604.091061.
32. Ocana-Macchi M, Bel M, Guzylack-Piriou L, Ruggli N, Liniger M, McCullough KC, et al. Hemagglutinin-dependent tropism of H5N1 avian influenza virus for human endothelial cells. J Virol. 2009 Dec;83(24):12947–55. http://dx.doi.org/10.1128/JVI.00468-09.
33. Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014 Jan;14(1):57–69. http://dx.doi.org/10.1016/S1473-3099(13)70286-X.
34. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011 Sep;146(6):980–91. http://dx.doi.org/10.1016/j.cell.2011.08.015.
35. Schmolke M, Viemann D, Roth J, Ludwig S. Essential impact of NF-kappaB signaling on the H5N1 influenza A virus-induced transcriptome. J Immunol. 2009 Oct;183(8):5180–9. http://dx.doi.org/10.4049/jimmunol.0804198.
36. Ehrhardt C, Ruckle A, Hrincius ER, Haasbach E, Anhlan D, Ahmann K, et al. The NF-kappaB inhibitor SC75741 efficiently blocks influenza virus propagation and confers a high barrier for development of viral resistance. Cell Microbiol. 2013 Jul;15(7):1198–211. http://dx.doi.org/10.1111/cmi.12108.
37. Yamaya M, Nishimura H, Shinya K, Hatachi Y, Sasaki T, Yasuda H, et al. Inhibitory effects of carbocisteine on type A seasonal influenza virus infection in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010 Aug;299(2):L160–8. http://dx.doi.org/10.1152/ajplung.00376.2009.