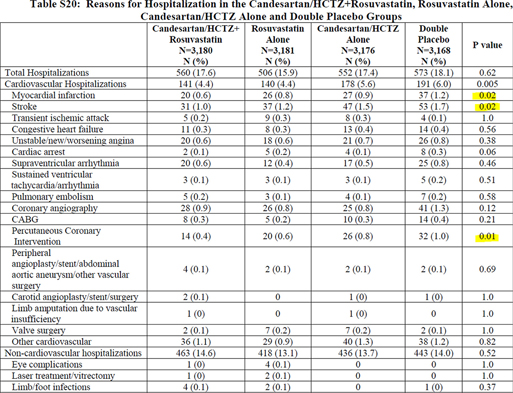
Figure 1. First table (despite being number 20) in the Supplementary materials showing the four groups randomized in HOPE-3. (Figure reproduced under license from the original publisher)
REVIEW ARTICLE
Mikael Rabaeus1, Paul V Nguyen2, Michel de Lorgeril3
1Groupe Médical de Chantepoulet, Genève, Switzerland; 2Centre Hospitalier Universitaire de Montréal, Montréal, Québec, Canada; 3Laboratoire TIMC-IMAG, UMR 5525 CNRS, Université Grenoble Alpes, Grenoble, France
Statin therapy is presented as a protection against ischemic heart disease (IHD) complications. As IHD is often a fatal disease, statins are thereby supposed to decrease cardiovascular mortality and increase life expectancy. However, these benefits are increasingly challenged in the medical community, the controversy being particularly intense when discussing the effects of statins in primary prevention and the consequences of statin discontinuation. Both primary prevention and treatment discontinuation have been recently used by investigators linked to the pharmaceutical industry to justify and boost prescription and consumption of statins and other cholesterol-lowering medications. We herein review some recent commercial data related to primary prevention with rosuvastatin and statin discontinuation and their respective effects on IHD and overall mortality rate. We conclude that (1) despite the recent hype raised by HOPE-3, the cholesterol-lowering rosuvastatin is likely not beneficial in intermediate-risk individuals without cardiovascular disease (primary prevention). This trial may even represent a typical example of how evidence-based medicine has been flawed in commercial studies. (2) Statin discontinuation does not lead to increased IHD and overall mortality, at least in the months following interruption of treatment. On the contrary, one might even conclude that statin discontinuation could save lives. One possible explanation of this apparently paradoxical finding is that statin discontinuers, in the same time they stop statin therapy, likely try to adopt a healthy lifestyle. Further studies are needed to confirm the real effects of statin discontinuation in various clinical conditions. In the meantime, it is not evidence based to claim that statin discontinuation increases mortality or saves lives.
Keywords: cholesterol; HOPE-3; myocardial infraction; rosuvastatin; statins
Statin therapy is presented as a protection against ischemic heart disease (IHD) complications. IHD being often a fatal disease, statins would thereby be expected to decrease overall and cardiovascular mortality and to increase life expectancy. However, an intense controversy has been growing about the true effects of statin therapy on IHD complications and mortality, including those described in randomized clinical trials (RCTs). The controversy is particularly intense around the effects of statins in primary prevention (1) and, by extension, around the consequences of statin discontinuation (2–5). Both elements are analyzed in this study.
On April 2, 2016, investigators of the Heart Outcomes Prevention Evaluation (HOPE)-3 trial reported the main results of a randomized double-blinded trial testing the effects of rosuvastatin (10 mg per day against placebo) on the risk of cardiovascular complications (6). The authors concluded that cholesterol lowering with rosuvastatin “resulted in a significantly lower risk of cardiovascular events than placebo in an intermediate-risk, ethnically diverse population without cardiovascular disease” (6). The associated editorial concluded that HOPE-3 “adds to the evidence supporting statin use for primary prevention” (7).
With all due respect, we think these statements should be seriously questioned. As recently underlined, the claims about efficacy (supposed to be high) and toxicity (supposed to be low) of statins are essentially based on RCTs published before 2005, which can be seriously criticized (1). Recent RCTs (published after 2005) are still equivocal, suggesting that even after 2005 basic methods of evidence-based medicine were still not fully and systematically respected (1). There are several ways of (intentionally or not) flawing RCT data, for instance, by not fully describing the raw data and/or only reporting partial data extracted from large database. Also, as the clinical files of randomized patients are quite easily accessible via Internet, unblinding is, although unproven, probably frequent. In consequence, health authorities are more and more precautious, and investigators are obliged to release increasing amounts of data, often performed in the form of “online supplementary materials.” Careful examination of all these released materials can provide information on the way the RCTs are conducted and analyzed. What about HOPE-3, the latest reported statin RCTs? Herein, we carefully examine all the available data about HOPE-3, and we will show relevant documents that can help understand the whole story.
The first major finding in HOPE-3 is that rosuvastatin clearly had no significant effect on mortality, whether all-cause [334 and 357 deaths in the rosuvastatin and placebo group, respectively] or cardiovascular [154 and 171 deaths] mortality (6). This is a major issue as mortality is one of the very rare end-points that cannot be “manipulated.” In addition, prolonging life expectancy is a patient’s first concern when having to consider taking a preventive medication “for life.” One should note that this absence of mortality benefit occurs despite several apparently favorable factors, such as a very significant low density lipoprotein (LDL) reduction, close to 30%; a huge sample size of 12,705 randomized patients; and a rather long follow-up, reaching a median duration of 5.6 years and allowing thereby what appears as a substantial number of deaths (n = 691), underlining that HOPE-3 was obviously not underpowered to detect an effect on mortality. HOPE-3, therefore, allows an initial conclusion that in the current state of knowledge, cholesterol lowering with statins does not prolong life in primary prevention as it does not prevent fatal cardiovascular complications. But then, one must of course also consider the prevention of nonfatal complications, resulting possibly in less patients suffering and in a positive cost-effectiveness ratio. This led us to carefully examine the risk of nonfatal complications associated with cholesterol lowering in HOPE-3.
When addressing the issue of nonfatal complications, we note that HOPE-3 investigators surprisingly chose to define two primary endpoints, called “first and second coprimary outcomes.” This suggests a semantic shift in itself as it indicates that there were two primary hypotheses. This is most certainly not in line with conventional methods in evidence-based medicine that are based on the principle of “one trial, one primary hypothesis, one single calculation of the required sample size and follow-up duration,” all in order to protect against an effect of chance (8).
Testing two primary hypotheses increases the risk of a type 1 error. To correct this, the authors state in the “Methods” section that the first co-primary outcome (CPO) was tested at a P value of 0.04 and the second at a P value of 0.02 (6). However, we believe that these corrected values could be insufficient to protect against type 1 error, all the more so as HOPE-3 investigators present the comparison of rosuvastatin with placebo as if there were only two randomized groups. There were in fact four groups as there was a second randomization to test an antihypertensive treatment (AHT, candesartan + HCTZ) with or without cholesterol lowering, both against placebo.
In summary, at least six primary hypotheses were tested in the same HOPE-3 trial:
In fact, investigators could also test whether combination of rosuvastatin and AHT is superior or inferior (if they want testing the occurrence of side effects) to rosuvastatin for both the 1st and the 2nd CPO eventually giving a total of eight primary hypotheses. The fact that these multiple hypotheses were not clearly formulated in the initial HOPE-3 article reporting the effects of rosuvastatin (6) raises major methodological and ethical issues. Many readers (in particular, very busy physicians) could think that in HOPE-3, rosuvastatin was compared with placebo and that there was only one primary hypothesis. Multiplication of primary hypotheses considerably increases the possibility of an effect of chance for one of them. Thereby, the P values to test the two CPOs should be much lower, probably close to 0.01 and 0.005, depending on chosen adjustments for multiple comparisons. This is the first reason that great caution must be exercised in evaluating the results about nonfatal complications, considering, in addition, that statistical significance is not the same as practical (clinical) significance.
As done by the authors, it is of course possible to present the results as a two-group rather than a four-group comparison, pooling the data from all the patients taking rosuvastatin (with or without antihypertensive) and comparing them with all the patients taking placebo (with or without antihypertensive). This means comparing two groups of roughly 6200 patients each, rather than four groups of 3100 each, obviously considerably increasing the power of the analyses. On the contrary, as mentioned by the investigators (6), comparing two groups (instead of four) remains only possible in the case of absence of interaction between cholesterol and blood pressure-lowering treatments. However, this cannot be stated as true as we see a clear interaction between treatments for at least three major components (myocardial infarction, stroke, and percutaneous coronary intervention) of the two CPOs (see Figure 1, reproducing Table S20 in the Supplementary materials) (6).

Figure 1. First table (despite being number 20) in the Supplementary materials showing the four groups randomized in HOPE-3. (Figure reproduced under license from the original publisher)
In the “Methods” section, the authors mention that the P values indicated in the last column of Table S20 (6) refer to the comparisons between the double-placebo group and the group combining cholesterol and blood pressure-lowering treatments. As such comparisons are post hoc analyses following ANOVA (with the four groups), it suggests (although not shown) significant interactions between the two treatments because post hoc tests are only allowed if ANOVA is significant, thus demonstrating interactions, which is indeed quite clear when looking at the numbers in the four groups (Figure 1).
It appears somewhat surprising to present the four randomized groups only as Supplementary materials (and only in the 20th table), as these findings show that it is imperative to present full data for the four groups and to only retain statistics that included the four groups. As the investigators chose not to present such four-group analyses, it appears that evidence-based medicine is not fully applied in HOPE-3 (6). We then examined the effects of rosuvastatin versus placebo by comparing the 3181 patients taking only rosuvastatin with the 3168 patients taking only placebo. We noted that the separate numbers of events in each of the four groups are quite small as well as the differences between groups, raising concern of the statistical significance, whatever the adjusted P value used to test any of the six or eight primary hypotheses. This definitely suggests that the results of HOPE-3 are not as clear as claimed by the investigators (6) and editorialists (7).
Actually, for the first CPO—the sum of cardiovascular deaths, nonfatal acute myocardial infarction (AMI), and stroke—as provided by the same table listing the results, the main manuscript states that there were 304 and 235 events in the placebo and the rosuvastatin group, respectively. However, these numbers differ from the sum of individual events constituting the 1st CPO, that is, 171 + 69 + 99 (total 339, placebo group) and 154 + 45 + 70 (total 269, rosuvastatin). This strongly suggests that the same numbers were not used in the main manuscript Table 1 and in the statistics, which, as already noted for the presentation of two instead of four groups, is misleading the readers. We obviously express serious concerns about this.
Another important issue lies in the documentation and adjudication of nonfatal events in HOPE-3. As an example, the definition and diagnostic conditions of AMI are somewhat curious. We read (once again in the Supplementary Appendix only) that “definite non-procedural AMI” could be diagnosed in case of “cardiac ischemic symptoms lasting at least 20 minutes, determined by the site investigator to be secondary to ischemia.” Although this is not the “usual” definition of AMI, it could be acceptable only if the double blinding is perfectly respected, which is likely not the case as discussed below. Also questionable is the definition “in cases of missing cardiac biomarkers” of the “probable non-procedural related myocardial infarction” as described in the Supplementary Appendix. This is not acceptable: when major markers of AMI are missing, this endpoint cannot be validated, especially if the double blinding is not fully respected and in the context of a commercial trial with major financial stakes involved. Such an absence of clear undebatable definitions of nonfatal cardiac complications in HOPE-3 is a major issue for several other reasons. For instance, were these hypothetical endpoints all included in the two CPOs? This was not made clear in the main article (6) or in the Canadian Journal of Cardiology article supposed to give more information about the methods used in HOPE-3 (9).
This issue is all the more important as with modern medicine, patient’s health records—especially electronic ones—are easily accessible, including patients participating in a trial. This clearly threatens the double blinding in any study testing the effect of cholesterol lowering, as discussed previously (10): lab records of changes in blood cholesterol can show whether patients are receiving the cholesterol-lowering statin or placebo. Such an unblinding can result in biasing the trial, leading to make the statin look more beneficial than it actually is (1, 10).
As an example, we reproduce in Figure 2 a real-world document, easily obtained by one of the authors from the hospital medical data file of one of his patients participating in HOPE-3. The sustained drop in a participant’s LDL measurements from 3.5 mmol/L to 1.5 mmol/L strongly suggests his allocation to rosuvastatin. Unblinding is a major issue in the process of documenting and adjudicating nonfatal cardiovascular complications. Knowing whether a patient is receiving a supposedly superior treatment (compared with placebo) or not may significantly influence the way that the physicians report (and interpret) symptoms. In addition, if the patients themselves are unblinded, it will obviously influence the way they report possible symptoms. As an example, in case of light or atypical symptoms, patients might be simply reassured and not hospitalized for careful follow-up and investigation if cholesterol is low (and thus patients thought to receive a protective statin). By contrast, the same patients with high cholesterol (thought to receive a placebo) may be rapidly admitted to the coronary care unit and subjected to full investigation including coronary angiography and possibly to revascularization, depending on various subjective or objective parameters. Considering the very approximate definitions of nonfatal cardiac complications used in HOPE-3 as described above—with a particular emphasis on the case of missing biomarkers in the diagnosis of myocardial infarction added to the possibility for local investigators to decide whether symptoms are secondary to ischemia or not—the probability of having a spurious difference between groups for nonfatal events is very high. All the more in a commercial trial with most local investigators financially linked to the sponsor. These considerations taken together, we feel that HOPE-3 does not raise confidence in the internal and external validity of the reported data.
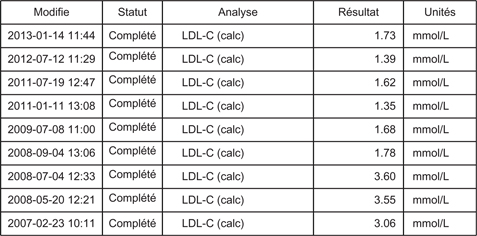
Figure 2. A real-world document extracted from the data file of a patient randomized in HOPE-3.
Yet another issue putting in question the validity of the results is evident when considering Figure 3 reproducing the graph S9 (6) (once again only provided in the Supplementary Appendix) showing the cumulative incidence of CPO 1 in the four separate groups. One notices no difference during the first 3 years of treatment. In particular, the red (rosuvastatin alone) and the blue (double placebo) curves clearly run together. The blue curve starts separating only somewhat around year 4 and then increasingly after year 6. At this time, the patient population only numbers around 1000, falling to about 250 during year 7 resulting in very few endpoints at these time points. Authors indicate statistics (hazard ratios and P values) for the comparisons between double placebo and “dual active treatment,” while what we do need are statistics on the comparison between placebo alone and rosuvastatin alone. What is the P value of the log-rank test comparing the two survival curves? Is that P value actually statistically significant after adjustment for multiple (n = 6 or n = 8) primary hypotheses? It is clearly allowed to doubt.
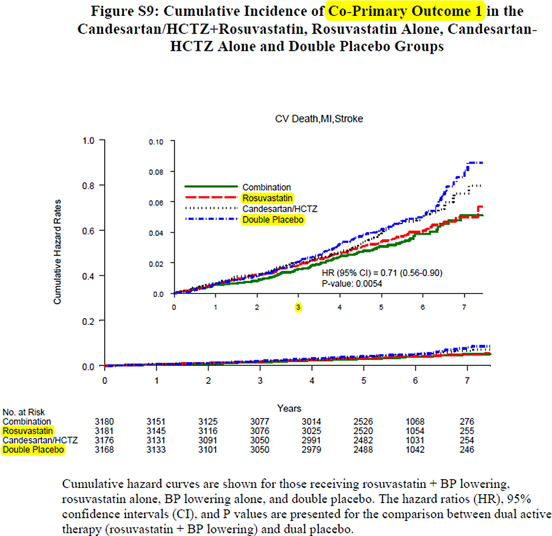
Figure 3. Reproduction of Figure S9 in the Supplementary materials. (Figure reproduced under license from the original publisher)
This is reinforced by the fact that the numbers of events are very small, nonfatal AMI and stroke in S20 (6) show a difference of 27 (37 vs. 26 for AMI and 53 vs. 37 for stroke). For cardiovascular death (Figure 4 reproducing Table S18 in the Supplementary Appendix of HOPE-3) (6), the difference between rosuvastatin alone and double placebo is 12 (91 vs. 79). It means that for the CPO 1, the difference between rosuvastatin alone and double placebo gives a total of 39 (12 + 27). Very clearly, whatever the adjusted P value used to compare the two groups, we think that it is highly likely that the difference is not statistically significant.
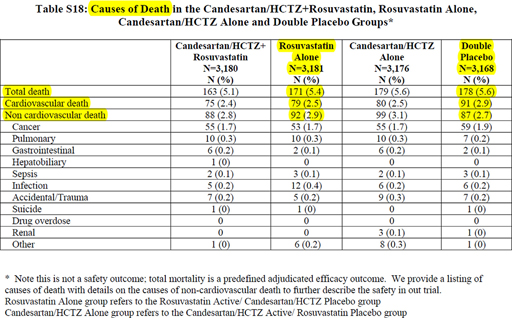
Figure 4. Reproduction of Table S18 in the Supplementary materials provided in the HOPE-3 trial. (Figure reproduced under license from the original publisher.)
Finally, we must remember the importance of reasoning also as medical doctors and not only as statisticians. Even if we naively accept the accuracy of the data in HOPE-3 as they are reported, the real significance is that one has to treat 3181 patients with 10 mg rosuvastatin for about 5.6 years to avoid 39 fatal and nonfatal cardiovascular complications, or 7 events per year. This corresponds to an number needed to treat (NNT) of 457 patients per year, which is of strictly no practical significance, especially when considering the direct and indirect costs as well as the adverse reactions. Put differently, treating 1000 patients for 1 year with rosuvastatin, one would avoid at best one nonfatal event (stroke or AMI) with no effect on life expectancy and no benefit to be expected during the first 3 years, as clearly shown on Figure 3.
In summary, despite efforts to claim the opposite, HOPE-3 appears to be a negative trial (when it comes to possible benefits of rosuvastatin) in terms of statistical and clinical significance. In addition, the main reported data are not clinically consistent, and the HOPE-3 trial is thereby misleading. Putting it more bluntly, HOPE-3 could represent a good illustration of the way “evidence-based medicine is hijacked,” according to the description recently given by Ioannidis (11). We thereby conclude that HOPE-3 does not raise any new hopes for the use of statins in primary prevention.
Recently, another argument was put forward to defend the usefulness of statin treatment, namely, that statin discontinuation increases overall and/or cardiovascular mortality. This could only mean that statins save lives (2–5). This assertion followed the publication of two large reviews recently published supporting the efficiency of cholesterol lowering and statin therapy (12, 13). One notes that most of the authors have significant links with the statin industry, which might have contributed to the surprising selection of the trials included in the meta-analyses (12, 13). As in previous commercially influenced reviews, the authors emphasize both the efficacy and safety of statins without discussing any evidence-based argument to the contrary. In addition, in the same British journal issue, the editorialist concurs with all these views, also putting forward that recent controversies over the efficacy and safety of statins may have harmed the health of thousands of people in UK (14).
This would be due to the fact that some patients stopped their statin treatment following controversies, and according to these authors, statin discontinuation will result in increased mortality. As, very rightly, both the authors (of these recent reviews) and the editorialists state that only evidence-based medicine should determine therapy for the prevention of cardiovascular disease (12–14), we set out to determine whether this had been applied when claiming that statin discontinuation invariably leads to increased mortality.
Before discussing statin discontinuation, it is important to make the difference with statin nonadherence. Medication nonadherence is a complex construct that can be simplified as poor execution by patients of the medical prescription. This includes total or partial lack of compliance in taking medication according to dosing regimen, leading to doses being reduced, delayed, or omitted. Nonadherence usually starts with the beginning of the treatment. There are many causes for nonadherence, including patient misunderstanding of the treatment goals, low education level, low financial income, and many others. Importantly, nonadherence is often associated with poor or even harmful lifestyle that may increase the risk of many acute or chronic diseases. As a consequence, it is often difficult to decide whether poor prognosis of certain IHD patients results from harmful lifestyle or statin nonadherence. In summary, observational studies investigating the effects of statin nonadherence cannot prove anything about causality, in particular because of the above described “healthy user bias.” In the recent medical literature, treatment discontinuation is rather used as patient’s decision to stop taking medication following a scientific, medical, or media controversy; and this decision may lead to transient, partial, or total interruptions in drug action (2–5). Contrary to nonadherence, statin discontinuation may reflect a mature decision by patients having doubts regarding the efficacy and safety of their treatment. According to some authors, the relationship between statin discontinuation and prognosis is less contaminated by confounding factors such as social, professional, and lifestyle factors, in particular the so-called healthy user bias (2–5).
If statin therapy actually saves lives, discontinuing this therapy obviously raises the question of thereby provoking an increased risk of fatal IHD complications (2–5). So far, only extrapolations and calculations have supported this concern (2–5). For instance, a retrospective Danish study estimated the absolute “increase” in total mortality to be 1.1% in the 10.5 years following statin cessation (3). One should note that the two mortality curves diverge during the first 4–5 years but are, thereafter, parallel and even converging during the last years of follow-up. This very small difference in mortality is not sufficient to raise concern, even more as results of such a study can also be influenced by several confounding factors, including the “healthy adherer effect” that was indeed mentioned by the authors (3). What does this mean? Simply that people who stop treatment by their own single initiative, that is, in the absence of any public controversy, are likely to be less “healthy” than those who continue to adhere closely to statin prescription or that people who stop treatment also stop visiting their attending physician do not wish adopting a healthy lifestyle—stop smoking, exercise more, adopt a protective healthy diet, for instance—and develop diseases that are independent of cholesterol levels and adhesion to statin treatment.
A British study calculated that the number of excess deaths in the UK, during the 10 years following statin cessation following the 2013 controversy, could be estimated at 2000 (4). This estimation is purely calculated and does not rely on truly observed mortality figures. Recently, a more objective approach of the issue was attempted (5). As the Danish and British authors cited above, these French investigators confess strong links to the pharmaceutical industry, and their data should be considered with caution (5). Using a 1/97th representative sample of the population covered by the French national healthcare insurance system, the authors report that following an intense controversy about statins in early 2013 in France, a 50% increase in statin discontinuation occurred during the year 2013 compared with 2012 and 2011. In the same period of 2013 (9 months), they recorded a 21% increase of mortality—about 80 extra deaths—in their sample (5). Extrapolating to the total French population, they calculated that controversy over statins may have provoked around 10,000 extra deaths in France during the year 2013 compared with 2012 and 2011, representing a “health tragedy that led to intense comments in popular media.”
However, subsequent publication of nationwide mortality rate in France for the year 2013 does not confirm the reality of this finding (15). This is a critical issue as national statistics are true and not “calculated” numbers. Very clearly, French mortality statistics show that no “health tragedy” occurred in 2013 in France (Table 1). As expected in a country where the total population is growing and ageing, both small yearly fluctuations and a slight trend toward an increased total number of deaths occurred between 2009 and 2013, but no increase in 2013 compared with 2012. On the contrary, there were 2000 deaths less in 2013 compared with 2012 representing an unexpected break in the ascending curve. Total cardiovascular and cerebrovascular deaths were either stable or decreased in 2013 compared with 2012. Even more significant, while the yearly average of cardiovascular deaths over years 2009–2012 was 141,500, it fell to 138,900 in 2013 suggesting that 2600 lives might have been “saved” in 2013 compared with previous years. Likewise, cerebrovascular deaths slightly decreased from a 2009–2012 average of 31,900 to 31,600 in 2013.
Regarding specifically IHD deaths, those supposed to be mainly reduced by statins, one observes again a decrease in 2013 (33,400 deaths) compared with 2012 (34,600) or the 2009–2012 average (35,200). We note (as shown in Table 1) that the decreased rate of fatal IHD tended to flatten in the years preceding 2013 (−1200 between 2009 and 2010, −900 between 2010 and 2011, and +200 between 2011 and 2012), leading to expect no decrease or even an increase between 2012 and 2013. On the contrary, there was an unexpected new decrease (−1200) in 2013 indicating that if statin discontinuation had played a role in the rate of fatal IHD in 2013, it was by preserving lives.
Nationwide mortality data are complex and should be cautiously interpreted. From the 2013 year data, however, we can safely conclude that statin discontinuation did not increase mortality in the short term, that is, within the months following discontinuation. In other words, no “health tragedy” occurred. The data could even suggest that statin discontinuation may reduce IHD and overall mortality and may have saved about 2000 lives in 2013 in France. It could of course be argued that the effect of statin discontinuation in 2013 on mortality would have been delayed to 2014. However, preliminary data from the French National Statistics System for the year 2014 do not confirm this possibility: overall mortality in 2014 is very close to that of 2013, confirming a slow decrease (16).
Obviously, these somewhat surprising data might be an effect of chance. Nevertheless, they clearly show that one must remain very cautious when extrapolating observational data, and thereby, they confirm that evidence-based medicine is fundamentally important. This being said, it does raise an interesting question: could a reduction of IHD mortality in the months following statin discontinuation be biologically plausible? Considering that statins were stopped by patients influenced by a controversy about statin therapy, they may in the same process also have decided that lifestyle changes are more effective than medication to protect health. We know that statins can be at the origin of muscle pain and fatigue. Patients also report sleep disorder when taking statins, inducing fatigue and deterring from exercise. Thereby, statin discontinuation, by alleviating sleep disorders, fatigue and muscle pain, may result in increased physical activity that has been clearly established as diminishing risk of fatal IHD (17). Indeed, the relationship between fatigue and mortality was recently confirmed in a study from UK that investigated a population-based cohort of 18,101 men and women, of which 4397 died during a mean follow-up of 16.6 years (18). The global mortality risk ratio was 1.4 when comparing those individuals with the highest level of fatigue with those with the lowest level. This mortality difference was most specifically observed for cardiovascular deaths.
Another important factor lies in the fact that many studies report that statins significantly increase insulin resistance and diabetes severity (19, 20). Sudden cardiac death (SCD) represents one of the most frequent causes of death in patients with diabetes, glucose intolerance, or insulin resistance (21, 22). However, in contrast to non-diabetic patients, global SCD risk has not been significantly reduced in diabetic patients, despite improvements in the treatment of IHD as well as of diabetes (21, 22). Statin discontinuation, by diminishing diabetes severity and improving insulin sensitivity, may, therefore, reduce the risk of fatal IHD through reduction of SCD risk.
Moreover, if patients decide to stop statin therapy after a public media episode of statin controversy, it is quite likely that some of them might decide to improve their diet as they stop statins. A healthy diet has been clearly established as preventing fatal IHD (23). Very simple changes can greatly reduce risk of fatal IHD and SCD (24). For instance, patients could decide to increase their consumption of fatty fish and thereby correct marine omega-3 fatty acid deficiency or insufficiency, which are known to be significant factors of fatal IHD and SCD (24). When they stop statins—which are, in addition, suspected to enhance omega-3 deficiency and to inhibit the protective effect of omega-3 (25)—and replace one meal of meat every week with a fatty fish meal, they would be achieving a significant reduction in fatal IHD risk (23–25).
Finally, some “discontinuing” patients could decide to stop smoking. Many patients taking statins actually keep on smoking because they think they are protected by statins. When they understand that statins are not protective at all, they stop treatment and, in the same breath, they stop smoking. Smoking acutely increases vasoconstriction and platelet reactivity (26). In consequence, the combined interruption of statin treatment and smoking inevitably results in rapid reduction of coronary thrombosis risk and fatal IHD.
Taken altogether, and contrary to current beliefs, statin discontinuation may not only not result in mortality increase, but it could even have favorable clinical effects. Obviously, our explanation of a possible positive effect is not evidence based. We put it forward with the aim of showing that a mortality reduction following rapidly after statin discontinuation can be envisaged. All the more, as it has been shown that lifestyle modifications show beneficial effects very quickly.
Scientists should seriously examine the issue in the near future by investigating the real effects of statin discontinuation rather than making dubious extrapolations and calculations. In the meantime, patients and physicians ought to just as seriously consider whether statin therapy is useful in each particular case as statin discontinuation definitely does not seem to be associated with deleterious effects, at least in the months following discontinuation. In summary, it cannot be considered as evidence based to continue to claim that statin discontinuation increases mortality or that statin therapy saves lives.
We thereby confirm, in total agreement with recent statements (11–14), that evidence-based medicine must be the cornerstone of modern medicine. This applies to both those who encourage and those who discourage a wide prescription of statins, affecting hundreds of millions of people globally.
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.