COMMENTARY
The Dilemma of Oxidative Stress Personified by the Diprosopus 8-iso-Prostaglandin F2α and Prostaglandin F2α
Dimitrios Tsikas
Core Unit Proteomics, Hannover Medical School, Hannover, Germany
Abstract
In general, the term “oxidative stress” describes an imbalance between oxidants and antioxidants in favor of the oxidants. While antioxidant defense is widely accepted to involve both enzymatic and non-enzymatic reactions, oxidants are generally assumed to be produced by non-enzymatic processes involving chemically produced free radicals. However, many oxidants are also formed by numerous enzymes and proteins. The F2-isoprostane 8-iso-prostaglandin F2α (8-iso-PGF2α) and malondialdehyde (MDA) are widely used as biomarkers of oxidative stress, although there is evidence that both 8-iso-PGF2α and MDA are also produced enzymatically from arachidonic acid by the action of cyclooxygenase (COX). On the contrary, there is also evidence that PGF2α is produced from arachidonic acid both by the action of COX and non-enzymatically. The duality of oxidative stress, personified by 8-iso-PGF2α and PGF2α, is a serious dilemma and demands new definitions and strategies from the scientists.
Keywords: 8-iso-prostaglandin F2α; arachidonic acid; cyclooxygenase inhibitors; oxidative stress; prostaglandin F2α
Received: 17 May 2017;
Accepted after revision: 04 June 2017;
Published: 28 June 2017.
Author for correspondence: Dimitrios Tsikas, Core Unit Proteomics, Hannover Medical School, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany. Email:
[email protected]How to cite: Tsikas D. The Dilemma of Oxidative Stress Personified by the Diprosopus 8-
iso-Prostaglandin F
2α and Prostaglandin F
2α. J Controversies Biomed Res 2017;3(1):11–15.
DOI:
http://dx.doi.org/10.15586/jcbmr.2017.20Copyright: Tsikas D
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
There is solid evidence that 8-iso-prostaglandin F2α (PGF2α) is produced both enzymatically and non-enzymatically in vivo in animals and in humans. This evidence is based on the inhibitory action of cyclooxygenase (COX) inhibitors and has been reported by many groups (1–3). For many decades, PGF2α has been thought to be exclusively formed from arachidonic acid by the catalytic action of COX. Yet, there is also solid evidence that PGF2α is an abundant non-enzymatic oxidation product of arachidonic acid (4). van't Erve et al. (5) proposed that the 8-iso-PGF2α/PGF2α molar ratio can be used to distinguish chemical from enzymatic lipid peroxidation. Yet, the key information that the isomeric prostaglandins 8-iso-PGF2α and PGF2α can be produced from arachidonic acid via enzymatic and non-enzymatic processes has not been considered by van't Erve and colleagues (5). It is worth mentioning that these authors have demonstrated that recombinant prostaglandin H synthase (PGHS) isoforms 1 (PGHS-1) and 2 (PGHS-2), also known as COX-1 and COX-2, respectively, catalyze the formation of 8-iso-PGF2α from free arachidonic acid (5). This finding confirms our recent finding on recombinant PGHS-1 and PGHS-2 (3). We also showed that PGHS-1 and PGHS-2 catalyze the synthesis of malondialdehyde (MDA) and 12-hydroxy-heptadecatrienoic acid (12-HHT), with MDA being a generally accepted biomarker of oxidative stress. Paradoxically, we found in the same study that reduced glutathione (GSH), the most abundant intracellular low-molecular-mass antioxidant, promotes the formation of 8-iso-PGF2α, MDA, and 12-HHT (3).
Can we distinguish enzymatic from non-enzymatic sources of PGF2α, 8-iso-PGF2α, MDA, and 12-HHT?
Generally, the proportion of enzymatic and non-enzymatic pathways contributing to PGF2α, 8-iso-PGF2α, MDA, and 12-HHT is unknown and is likely to vary greatly in biological systems. Alternative approaches to distinguish enzymatic and non-enzymatic pathways have been proposed. They involve the use of the molar ratio of 8-iso-PGF2α to another prostanoid such as the parent molecule arachidonic acid (6) or prostaglandin E2 (PGE2) (3, 7) (Figure. 1). PGE2 is arbitrarily assumed to being exclusively produced from arachidonic acid by PGHS. Yet, the exclusive source of PGHS for PGE2 remains to be demonstrated. Noteworthy, 8-iso-PGE2 is known to readily and spontaneously isomerize to PGE2, so that the origin of PGE2 and its metabolites measured in biological samples are eventually uncertain. This may also apply to prostacyclin (PGI2) and thromboxane A2 (TxA2) (8). These issues underline the Gordian nature of oxidative stress.
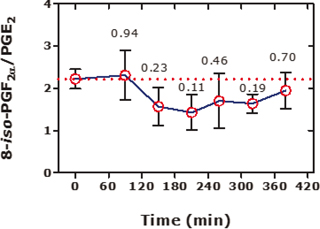
Figure 1. Effects of N-acetylcysteine (NAC) on the urinary 15(S)-8-iso-PGF2α/PGE2 molar ratio in eight healthy subjects (4 females, 4 males) upon a single oral intake of 600 mg NAC. NAC was administered immediately after collection of the first urine samples at time 0 h. Urinary excretion rates ranged between 25 and 183 nmol/mol creatinine for 15(S)-8-iso-PGF2α and between 65 and 315 nmol/mol creatinine for PGE2. Data are shown as mean ± SEM. The numbers indicate the Mann–Whitney test P values. This Figure was constructed with data reported in Figure 1 panels (C) and (D) of a previous study (7).
Analytical and methodological issues contributing to uncertainty and controversies
Two important issues of the proposal by van't Erve et al. (5) are worthy of discussion: (a) The use of different kinds of biological samples in which 8-iso-PGF2α and PGF2α were analyzed and the different pre-analytical and analytical methods applied. (b) The consideration of data obtained under very diverging conditions to distinguish chemical from enzymatic generation of 8-iso-PGF2α and PGF2α and to quantify the individual contributions.
In general, measurement of primary prostanoids in blood (plasma and serum) is not recommended because of their abundant ex vivo formation (discussed in Ref. [9] of this article). In healthy humans, plasma PGF2α concentration ranges between 2 and 11 pg/mL (9). The concentration of free, non-esterified 8-iso-PGF2α in plasma of healthy non-smoking volunteers is of the order of 3–10 pg/mL as measured by two highly specific methods, that is, by the combination of immunoaffinity chromatography (IAC) column extraction of 8-iso-PGF2α with GC-MS/MS (9). van't Erve et al. reported 5–10 times higher plasma concentrations (range, 50 and 110 pg/mL) for 8-iso-PGF2α and PGF2α in healthy humans (5). This discrepancy challenges the specificity of the LC-MS/MS methodology as well as the reliability of measuring plasma concentrations of 8-iso-PGF2α, PGF2α, and other primary prostanoids which circulate in blood at concentrations generally below 10 pg/mL (8, 9). The low concentration range is a big analytical challenge for every analytical methodology presently available including GC-MS/MS, but much more so for LC-MS/MS (9). As a consequence of this, the use of molar ratios such as 8-iso-PGF2α to PGF2α in plasma is likely to lead to disputable results. Similar difficulties may also apply to other biological samples including urine because of different origin and metabolism of 8-iso-PGF2α and PGF2α excreted in the urine. In addition, PGHS inhibitors may exert distinctly different effects on 8-iso-PGF2α in humans and in widely used experimental animals such as rats (8). Implementation of IAC techniques for the specific separation and isolation of 8-iso-PGF2α and PGF2α from the remaining potentially interfering 62 F2-prostaglandins prior to LC-MS/MS or GC-MS/MS analysis is recommended.
The input of data obtained under very different conditions into a formula such as that proposed by van't Erve et al. (5) for distinguishing between chemical and enzymatic generation of 8-iso-PGF2α and PGF2α, as well as for quantifying the relative contribution of the enzymatic and non-enzymatic pathways, is problematic for the following reasons: (a) Data on 8-iso-PGF2α obtained from rats and humans are hard to compare (see Ref. [8]). (b) Results obtained from the use of recombinant PGHS enzymes and intact cells are also difficult to compare. (c) The use of the free radicals generating chemical 2,2´-azobis-2-methyl-propanimidamide dihydrochloride (AAPH) at the very high concentration of 10 mM compared to arachidonic acid which was used at the physiologically relevant concentration of about 20 µM is challenging. (d) The very long incubation times (e.g., 5–24 h) in some of the experiments performed by van't Erve et al. (5) may not correctly indicate the actual contribution of chemical and enzymatic peroxidation of arachidonic acid. Finally, (e) the measurement of 8-iso-PGF2α and PGF2α in blood is not suitable for the reasons discussed above. With respect to the very long incubation time of at least 5 h, it should be emphasized that “oxidative stress” occurs immediately upon addition of arachidonic acid to PGHS (3) under experimental conditions very similar to those used by van't Erve et al. (5). For instance, in the presence of recombinant PGHS-1 or PGHS-2 and arachidonic acid (10 µM), the thiols glutathione (100 µM) or L-cysteine (100 µM) are rapidly oxidized to their disulfides in a stoichiometry of about 1:1 with respect to arachidonic acid, and the reaction is already completed after about 20–30 min of incubation at 37°C (3).
PGE2 is the major reaction product that is generated from arachidonic acid by recombinant PGHS-1 and PGHS-2 (3). In the absence of thiols, synthetic PGG2 decomposes spontaneously to a minor extent to PGE2 in a manner independent of hematin and phenol (Figure 2A); the latter are recommended and commonly used in activity assays of isolated and recombinant PGHS-1 and PGHS-2. Under the same conditions, synthetic PGH2 decomposes mainly to PGE2 to a much higher extent of 70%–80% (Figure 2A). In the presence of the thiol N-acetyl-L-cysteine (Figure 2B) or glutathione (Figure 2C), the decomposition of PGH2 to PGE2 decreases, while the formation of F2-prostanoids such as 8-iso-PGF2α, 9β,11α-PGF2α, 15-keto-PGF2α, and PGF2α increases several fold. On the contrary, thiols seem not to affect decomposition of synthetic PGG2. Similar effects are also seen in the presence of recombinant PGHS-1 or PGHS-2, yet in the absence of synthetic PGG2 or PGH2, in a manner depending on the incubation time (Figures 3 and 4). These observations indicate that certain changes in experimental conditions may affect significantly the pattern of prostanoids and consequently of their molar ratios (Figure 4). Diverging results are also obtained using PGHS inhibitors with different modes of action such as acetylsalicylic acid (irreversible inhibitor) or diclofenac and indomethacin (both reversible inhibitors) (3).
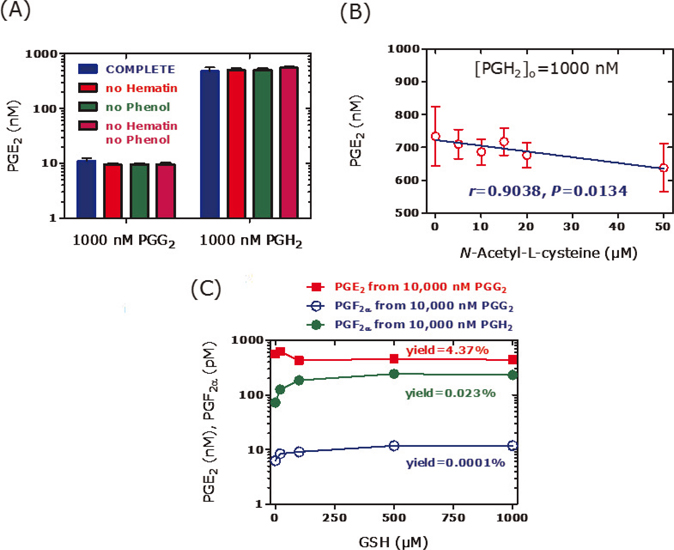
Figure 2. Formation of PGE2 and PGF2α from synthetic PGG2 and PGH2 in buffer at 37°C after incubation for 10 min. (a) Each 1000 nM of PGG2 and PGH2 were incubated in complete buffer (2 mM phenol, 1 µM hematin), as well as in the absence of phenol, of hematin or of both. (b) Effect of various concentrations of N-acetyl-L-cysteine (0–50 µM) on the formation of PGE2 from synthetic PGH2 used at a nominal concentration of 1000 nM in complete buffer. (c) Effect of reduced glutathione (GSH) used at various concentrations (0–1000 µM) on the formation of PGE2 from synthetic PGG2 and PGH2 used at a nominal concentration of 10,000 nM each in complete buffer. Experimental conditions and prostanoids analysis were as described elsewhere (3). Data are shown as mean and standard deviation of two independent experiments.
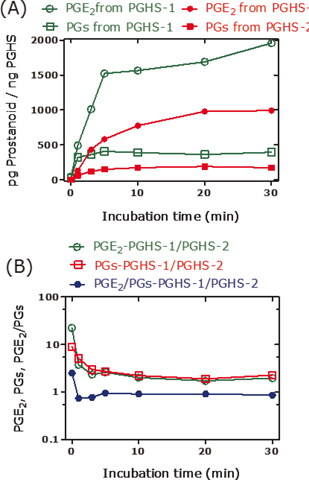
Figure 3. PGE2 and prostanoids (PGs, PGD2+15-keto-PGF2α+9ß,11α-PGF2α+PGF2α+8-iso-PGF2α) formed in incubation mixtures of arachidonic acid (10 µM), recombinant PGHS-1 (5 U), or recombinant PGHS-2 (5 U) at 37°C for the indicated time points. (a) Amount of PGE2 and PGs per pg PGHS-1 or pg PGHS-2. (b) Molar ratio of PGHS-1- to PGHS-2-derived PGE2, PGs, and their molar ratio. Experimental conditions and prostanoids analysis were as described elsewhere (3).
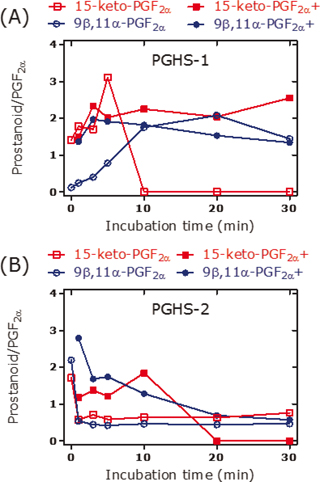
Figure 4. Molar ratio of the prostanoid 15-keto-PGF2α or 9ß,11α-PGF2α to PGF2α in incubation mixtures of arachidonic acid (10 µM) and (a) recombinant PGHS-1 (5 U) or (b) recombinant PGHS-2 (5 U) in the absence or in the presence (indicated by the symbol +) of N-acetyl-L-cysteine (100 µM) at 37°C for the indicated time points. Experimental conditions and prostanoids analysis were as described elsewhere (3).
Conclusion
The scientific research area of oxidative stress is highly challenging, and the role attributed to free radicals in the development of chronic diseases and aging process is overestimated (10). Many of the biomarkers being presently used to quantify oxidative stress, including 8-iso-PGF2α and MDA, can be generated both by enzymes and free radicals or by non-radical reactive oxygen/nitrogen species, which, in turn, are produced both by enzymes and chemicals especially including transition metal ions. Endogenous substances such as GSH can modulate the fate of intermediates (i.e., PGG2 and PGH2) in favor of 8-iso-PGF2α and PGF2α and at the cost of other prostaglandins such as PGE2 and PGD2α, the kinetics and yield of reaction products. Distinguishing “enzymatic” from “non-enzymatic” pathways/reactions that lead to 8-iso-PGF2α, PGF2α, and MDA by means of a combination of in vitro and in vivo studies is extremely difficult, if not impossible. The focus in this area should be on those F2-prostaglandins which, in theory, are unlikely to be produced from arachidonic acid by the catalytic action of PGHS and/or of other enzymes/proteins. Some of those F2-isoprostanes have been sporadically investigated and used as biomarkers of oxidative stress in the past. Studies comparing 8-iso-PGF2α, a type III (15 series) F2-isoprostane, with F2-isoprostanes of type IV (8 series), type V (12 series), or VI (5 series) will provide valuable information about the nature of oxidative stress, notably of lipid peroxidation. COX inhibitors and antioxidants, most notably thiols such as N-acetylcysteine, are likely to generate different results in vitro using recombinant PGHS enzymes, in cells, and in vivo in animals and in humans for many different reasons which have not been fully recognized thus far.
8-iso-PGF2α has been recently declared as “the best biomarker of oxidative stress” in humans, and the 8-iso-PGF2α/PGF2α molar ratio has been proposed as a quantitative measure of lipid peroxidation (5). Yet, scientifically sound evidence has not been provided. The dual nature of oxidative stress and the diprosopus 8-iso-PGF2α, PGF2α, and MDA are likely to be objectives of research of scientist generations to come.
Conflict of interest
The author declares no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
-
Pratico D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of the isoprostane, 8-epi prostaglandin F2 alpha. J Biol Chem. 1995 Apr 28;270(17):9800–8. https://dx.doi.org/10.1074/jbc.270.17.9800
-
Klein T, Reutter F, Schweer H, Seyberth HW, Nüsing RM. Generation of the isoprostane 8-epi-prostaglandin F2alpha in vitro and in vivo via the cyclooxygenases. J Pharmacol Exp Ther. 1997 Sep;282(3):1658–65.
-
Tsikas D, Suchy MT, Niemann J, Tossios P, Schneider Y, Rothmann S, et al. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F2α. FEBS Lett. 2012 Oct 19;586(20):3723–30. https://dx.doi.org/10.1016/j.febslet.2012.09.001
-
Yin H, Gao L, Tai HH, Murphey LJ, Porter NA, Morrow JD. Urinary prostaglandin F2alpha is generated from the isoprostanes pathway and not the cyclooxygenase in humans. J Biol Chem. 2007 Jan 5;282(1):329–36. https://dx.doi.org/10.1074/jbc.M608975200
-
van 't Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic Biol Med. 2015 Jun;83:245–51. https://dx.doi.org/10.1016/j.freeradbiomed.2015.03.004
-
Halliwell B, Lee CY. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid Redox Signal. 2010 Jul 15;13(2):145–56. https://dx.doi.org/10.1089/ars.2009.2934
-
Tsikas D, Niemann J, Flentje M, Schwarz A, Tossios P. N-Acetylcysteine (NAC) inhibits renal nitrite and nitrate reabsorption in healthy subjects and in patients undergoing cardiac surgery: Risk of nitric oxide (NO) bioavailability loss by NAC? Int J Cardiol. 2014 Nov 15;177(1):30–3. https://dx.doi.org/10.1016/j.ijcard.2014.09.109
-
Bachi A, Brambilla R, Fanelli R, Bianchi R, Zuccato E, Chiabrando C. Reduction of urinary 8-epi-prostaglandin F2 alpha during cyclo-oxygenase inhibition in rats but not in man. Br J Pharmacol. 1997 Aug;121(8):1770–4. https://dx.doi.org/10.1038/sj.bjp.0701321
-
Tsikas D, Zoerner AA. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: A historical retrospect and a discussion. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Aug 1;964:79–88. https://dx.doi.org/10.1016/j.jchromb.2014.03.017
-
Vitetta L, Coulson S, Linnane AW. Reactive oxygen species in disease: Rebuttal of a conventional concept. J Controvers Biomed Res. 2015 Sep;1(1):23–27. https://dx.doi.org/10.15586/jcbmr.2015.7



