ORIGINAL ARTICLE
Does Oxidative Stress Change during Orthotopic Liver Transplantation?
Dimitrios Tsikas
Core Unit Proteomics, Hannover Medical School, Hannover, Germany
Abstract
In clinical ischemia/reperfusion injury, damage resulting from oxidative and nitrosative stress is generally considered crucial for graft functioning. Yet, there is reasonable doubt that modern clinical transplantation including orthotopic liver transplantation (OLT) is associated with elevation of oxidative and nitrosative stress upon organ reoxygenation. We measured two biomarkers of oxidative stress, 15(S)-8-iso-prostaglandin F2α (15(S)-8-iso-PGF2α) and cis-epoxyoctadecanoic acid (cis-EpOA), in human plasma during the entire time duration of OLT in nine patients suffering from end-stage liver disease. No considerable concentration changes of 15(S)-8-iso-PGF2α and cis-EpOA were observed in plasma, indicating lack of oxidative stress. Creatinine-corrected urinary excretion of 15(S)-8-iso-PGF2α in two urine samples collected 40–60 min and 60–240 min after reperfusion did not increase significantly. Previously, we found in the same patients that nitrosative stress, measured as 3-nitrotyrosine and 3-nitrotyrosinoalbumin, is elevated in the patients but did not change during OLT. 15(S)-8-iso-PGF2α, cis-EpOA, 3-nitrotyrosine and other widely used biomarkers of oxidative stress, notably malondialdehyde (MDA), are produced both by chemical and enzymatic reactions. This important issue is rarely considered in the area of oxidative and nitrosative stress. In addition, many widely used analytical approaches such as the total antioxidant capacity (TAC) assay to quantitate oxidative and nitrosative stress in health and disease and during clinical transplantation lack reliability. New basic concepts of oxidative and nitrosative stress need to be drafted and implemented in vitro as well as in vivo in experimental and clinical settings.
Keywords: analytical chemistry; arachidonic acid; COX; CYP; F2-isoprostanes; humans; oleic acid oxide; total antioxidant capacity
Received: 07 August 2017;
Accepted after revision: 21 September 2017;
Published: 01 November 2017
Author for correspondence: Dimitrios Tsikas, Core Unit Proteomics, Hannover Medical School, Carl-Neuberg-Strasse 1, DE-30625 Hannover, Germany. Email:
[email protected]How to cite: Tsikas D. Does oxidative stress change during orthotopic liver transplantation? J Controversies Biomed Res. 2017;3(2): 1–5
Doi:
http://dx.doi.org/10.15586/jcbmr.2017.23Copyright: Tsikas D.
License: This open access article is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0
Introduction
Assessment of oxidative and nitrosative stress is based on the measurement of their respective so-called biomarkers. Application of this approach to in vitro and in vivo conditions in humans revealed involvement of oxidative and nitrosative stress in numerous diseases (1). In patients suffering from end-stage liver disease, we measured higher concentrations of 15(S)-8-iso-prostaglandin F2α (15(S)-8-iso-PGF2α) (2) and 3-nitrotyrosine and 3-nitrotyrosinoalbumin (3) than in healthy subjects. 15(S)-8-iso-PGF2α and other F2-isoprostanes and 3-nitrotyrosine are generally considered reliable biomarkers of oxidative stress (lipid peroxidation) (1, 4) and nitrosative stress (5), respectively. On the basis of the general assumption that elevated biomarker concentrations in biological samples are indicative of elevated oxidative and nitrosative stress, our findings on 15(S)-8-iso-PGF2α, 3-nitrotyrosine and 3-nitrotyrosinoalbumin (2, 3) would suggest that liver diseases are associated with elevated oxidative and nitrosative stress. However, oxidative and nitrosative stress represent a considerable challenge in this area of research (1, 5), not least because both enzymatic and non-enzymatic sources including antioxidants such as reduced glutathione (GSH) commonly contribute to oxidative and nitrosative stress to a variable mostly of non-determinable extent. Thus, cyclooxygenase (6, 7) and myeloperoxidase are considerable contributors to 15(S)-8-iso-PGF2α and 3-nitrotyrosine, respectively. Another example of the dual nature of oxidative stress is cis-epoxyoctadecanoic acid (cis-EpOA). cis-EpOA can be produced from oleic acid enzymatically by many hepatic cytochrome P450 (CYP) isoforms (8). However, cis-EpOA can also be produced from oleic acid non-enzymatically (9, 10). In serum samples of six young females (age range 12–18 years) with suspected paracetamol poisoning (serum paracetamol concentrations: 132, 166, 185, 270, 766, and 921 μM), we measured cis-EpOA concentrations of 47, 3723, 65, 275, 319, and 300 nM but did not find any correlation between paracetamol and cis-EpOA concentrations (unpublished observations). In humans suffering from end-stage liver disease, we measured plasma concentrations of cis-EpOA and found it to be lower than that in healthy subjects (8). This finding may suggest diminished hepatic synthesis of cis-EpOA by CYP isoform and/or lacking elevated oxidative stress in liver disease. However, in plasma samples of the same patients, we found elevated 15(S)-8-iso-PGF2α, 3-nitrotyrosine, and 3-nitrotyrosinoalbumin concentrations (2, 3), suggesting elevated oxidative and nitrosative stress in end-stage liver disease. Because of the duality of oxidative stress and for many other reasons, notably including analytical shortcomings and pitfalls (1), the conventional concept of oxidative stress in human disease has been questioned (1, 11).
The total antioxidative capacity (TAC) of plasma is usually determined and used as a measure of oxidative stress instead of measuring individual biomarkers of oxidative stress. However, TAC assays have also several drawbacks and limitations (1, 12, 13). There is evidence that several clinical, metabolic, or physiological conditions, not necessarily correlated with oxidative stress, may contribute to TAC of the plasma, thus potentially leading to questionable conclusions regarding the status of oxidative stress (reviewed in Ref. (1)). Recently, we found that clinical ischemia/reperfusion studies in liver (3) and kidney and heart (14) do not result in elevation of nitrosative and oxidative stress challenge. In the present work, we report original data from the analysis of 15(S)-8-iso-PGF2α and cis-EpOA in plasma samples collected during OLT in previous study (15) and discuss them in the context of oxidative stress.
Materials and Methods
The characteristics of the nine patients with end-stage liver disease who underwent full-size OLT in standard technique without veno-venous bypass (7 males, mean age 45.7 ± 14.7 years; 2 females of 42 and 58 years of age) have been described in detail elsewhere (15). The study protocol was approved by the local ethics committee and the study was performed according to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all patients. Serial blood samples were taken from peripheral veins or from central venous catheters at the following time points: preoperatively, before anesthesia, after skin incision, 1 min before unhepatic clamping time, and 0, 5, 10, 20, 40, 60, 120, and 240 min after reperfusion. EDTA plasma was generated by immediate centrifugation (800 g, 5 min, 2 °C), aliquoted properly, and stored at −80 °C until analysis. Urine samples were collected preoperatively from spontaneous micturition (U1), 40–60 min after reperfusion (U2), and 60–240 min after reperfusion (U3) by means of urethral catheters. Urine samples were aliquoted properly and stored at −80 °C until analysis. The concentration of urinary free 15(S)-8-iso-PGF2α was corrected for creatinine excretion and is expressed as nanomole of analyte per mole of creatinine.
Free nonconjugated 15(S)-8-iso-PGF2α and total, that is, free+esterified 15(S)-8-iso-PGF2α, and free cis-EpOA were measured separately each in 1-mL plasma aliquots using deuterium labeled analogs of 15(S)-8-iso-PGF2α and cis-EpOA, which served as internal standard by GC-MS/MS as described elsewhere (9, 16). All GC-MS/MS analyses were performed on a Thermoquest TSQ 7000 triple-stage quadrupole mass spectrometer interfaced with a Thermoquest gas chromatograph model Trace 2000 which was equipped with a programmed temperature evaporation injector and an autosampler model AS 2000.
Results
In plasma, the concentrations of 15(S)-8-iso-PGF2α and cis-EpOA did not change statistically significantly during OLT (Figure 1A and 1B) and did not correlate with each other (r = 0.12, P = 0.72 considering all time points). Continuous concentration decays were observed for plasma 15(S)-8-iso-PGF2α and cis-EpOA upon liver reoxygenation, with the decrease in cis-EpOA concentration being more pronounced (Figure 1A and 1B). In this period of time, plasma 15(S)-8-iso-PGF2α and cis-EpOA concentrations failed statistical correlation (r = 0.63, P = 0.09) (Figure 1C). At the end of the OLT, the plasma concentrations of 15(S)-8-iso-PGF2α and cis-EpOA were very close to their values at the beginning of the OLT, yet without reaching normal values in healthy subjects (2, 3).
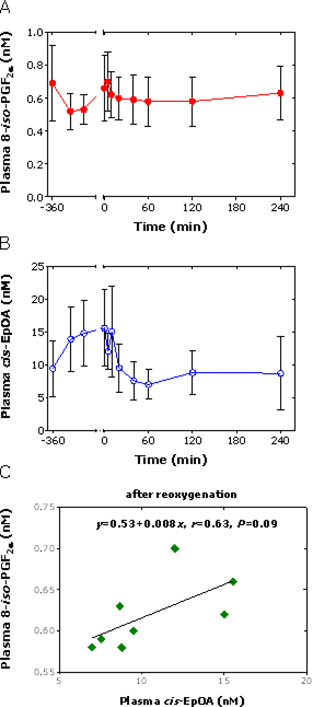
Figure 1. Time course of the plasma concentrations (mean ± standard deviation) of (A) total 15(S)-8-iso-PGF2α (free plus esterified forms) and (B) free cis-EpOA during full-size OLT in standard technique without veno-venous bypass in eight patients with end-stage liver disease. (C) Linear regression analysis between the circulating concentrations of 15(S)-8-iso-PGF2α and cis-EpOA after the liver reoxygenation including the time point 0 min. Time “0” indicates the time of liver reperfusion.
The creatinine-corrected excretion of 15(S)-8-iso-PGF2α increased from (mean ± standard deviation) 135 ± 83 nmol/mol (U1) to 204 ± 104 nmol/mol (U2) and to 186 ± 114 nmol/mol (U3), yet with the increases failing to reach statistical significance (P = 0.057 and P = 0.064, respectively) (Figure 2).

Figure 2. Creatinine-corrected excretion of free non-conjugated 15(S)-8-iso-PGF2α by nine patients who underwent OLT. Urine samples U1 were collected prior to anesthesia by spontaneous micturition. Urine samples U2 (40–60 min after reperfusion) and U3 (60–240 min after reperfusion) were collected by using urethral catheters.
Discussion
Two proteomic studies revealed that the liver proteome changes during OLT; modifications were found to include upregulation of proteins associated with energy metabolism, various metabolic pathways, oxidative-stress antioxidant response, and lipid metabolism) (17, 18). Unfortunately, the design of these studies did not allow to answer the question whether or not these modifications would finally result in elevation of oxidative and nitrosative stress. Previously, we found that the L-arginine/nitric oxide (NO) pathway and related L-arginine-involving pathways changed during OLT (15). The major change was abrupt and strong decay of plasma arginine concentration and concomitant abrupt increase in plasma L-ornithine concentration. This observation is best explained by assuming arginase-I release from the graft and conversion of L-arginine to L-ornithine activity upon liver reperfusion (15). The relatively long-lasting increase in plasma L-arginine concentration could be an indication of a deficiency in the ubiquitously required cofactor ATP which is also required for the synthesis of L-arginine from L-citrulline. This important issue has not been adequately considered in the past (discussed in Ref. 19).
In the same study, we observed no statistically significant changes in the plasma concentrations of 15(S)-8-iso-PGF2α (free+esterified) and cis-EpOA (free) during the entire period of the OLT. The greatest changes were observed for cis-EpOA of which the plasma concentration decreased continuously upon reperfusion. cis-EpOA can be formed by CYP-catalyzed epoxidation of oleic acid and the OLT patients of the present study had lower plasma cis-EpOA concentration than healthy subjects (8). As CYP-catalyzed synthesis of cis-EpOA requires the cofactor NADPH (8, 20), deficiency in NADPH after liver reperfusion may be an explanation for the continuous decay of plasma cis-EpOA concentration during OLT, which is analogous to the decrease in plasma L-arginine concentration presumably due to ATP deficiency (15), as ATP is required for the synthesis of L-citrulline, the precursor of L-arginine.
While the plasma concentration of 15(S)-8-iso-PGF2α did not change during OLT of the present study, the urine excretion of 15(S)-8-iso-PGF2α seems to have increased upon reperfusion. This observation may suggest elevation of oxidative stress (lipid peroxidation) upon liver reperfusion. However, it should be kept in mind that 15(S)-8-iso-PGF2α is also produced enzymatically by cyclooxygenase (COX) (4, 6, 7). It is worth mentioning that the excretion of 15(S)-8-iso-PGF2α and prostaglandin E2 (PGE2), a “classical” COX metabolite of arachidonic acid, also increased in patients undergoing cardiac surgery, both under placebo and upon N-acetylcysteine infusion (21).
The urinary excretion of the major urinary metabolite of 8-iso-prostaglandin F2α (IPF2α-III) was found to be higher in patients with end-stage liver disease than in healthy control subjects and to increase intraoperatively during OLT (22). These observations are in agreement with our observations on the parent compound 15(S)-8-iso-PGF2α. As no analyses of the 8-iso-prostaglandin F2α or of its metabolite in plasma were performed (22), it is not clear whether the increase in the urinary excretion of the metabolite seen intraoperatively is due to elevated excretion of the circulating 8-iso-prostaglandin F2α metabolite or due to elevated synthesis and metabolism of its precursor 8-iso-prostaglandin F2α during the OLT. 8-iso-Prostaglandin F2α synthesis in the study (22) is likely to have occurred by the mediation of COX, presumably by the inducible form COX-2.
In other studies, TAC of plasma and thiobarbituric acid-reactive substances (TBARS) including malondialdehyde (MDA) were measured 5 min before, 15 min after reperfusion, and at the end of the OLT (23). In that study, TAC values were found to decrease by about 10% after reperfusion and to reach the baseline value at the end of the transplantation; TBARS and TAC values did not correlate at each time point (23). In another study, OLT was reported to result in about twofold elevation of red blood cell MDA and plasma lipid peroxide levels 120 min after reperfusion (24). However, the clinical utility of plasma TAC assays is questioned because many factors including diet and altered metabolic pathways may falsely contribute to TAC independent of oxidative stress (1, 12, 13).
Finally, in some OLT studies, measurement of biomarkers of oxidative stress in exhaled breath condensate (EBC) during OLT has been proposed (25). However, the reported concentrations of H2O2 (70–150 mM), 8-iso-prostaglandin F2α (20–30 pg/mL), and NO (presumably nitrite+nitrate; 20–35 μM) in EBC are in part much too high (26, 27) and question the reported claims of elevation of oxidative stress upon reperfusion. The utility of 8-iso-prostaglandin F2α in EBC as a biomarker of oxidative stress in human disease remains to be demonstrated (26).
Conclusion
Oxidative stress is assessed by different means, which include quantitative determination of “established” biomarkers of oxidative stress and the measurement of the TAC of the plasma. Based on such approaches, oxidative stress was found to be involved in numerous diseases as well as in clinical ischemia/reperfusion injury including OLT. By measuring the “established” biomarkers of oxidative stress (i.e., 15(S)-8-iso-PGF2α), and nitrosative stress (i.e., 3-nitrotyrosine and 3-nitrotyrsoine albumin) using reliable gas chromatography-tandem mass spectrometry-based analytical approaches, we found that oxidative stress (present study) and nitrosative stress are elevated in patients with end-stage liver disease, but they do not change during OLT. In our study, we also used cis-EpOA, which is well established to be formed from oleic acid both by the catalytic action of several hepatic CYP isoforms and by oxidant species such as peroxynitrite. cis-EpOA was found at lower plasma concentrations in patients suffering from end-stage liver disease compared to healthy subjects and its plasma concentration seems to decrease upon reperfusion. These partially contradictory findings indicate that the traditional concepts of oxidative and nitrosative stress and the analytical approaches commonly used to measure their extent in health and disease and their changes during OLT seem to be a dead-end street. Obviously, new concepts and reliable analytical methods are required in in vitro and in vivo studies dedicated to oxidative and nitrosative stress. Special emphasis must be given to the dual nature of oxidative stress, which is the contribution of enzymes such as COX and CYP and of chemical oxidants such as hydrogen peroxide and peroxynitrite. Admittedly, generation and establishment of new concepts are formidable challenges and Sisyphean tasks. Regarding the analytical approaches, mass spectrometry is the most promising technique; however, it is not free of pitfalls (5) and must be used correctly both for low-molecular-mass and high-molecular-mass biomolecules (28). In this work, we focused on OLT. However, we believed that the issues discussed here are likely to hold true for heart and kidney transplantation, and more generally for human disease. And a last but not least important issue is that scientists, that is, authors, reviewers, and editors, need to learn from mistakes made in the past (29).
Conflict of Interest:
The author declares no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–81. http://dx.doi.org/10.3109/10408360903142326
- Tsikas D, Rode I, Becker T, Nashan B, Klempnauer J, Frölich JC. Elevated plasma and urine levels of ADMA and 15(S)-8-iso-PGF2α in end-stage liver disease. Hepatology. 2003;38:1063–4. http://dx.doi.org/10.1002/hep.1840380440
- Tsikas D, Frölich JC, Klempnauer J, Becker T. Nitrosative stress does not change during orthotopic liver transplantation. Liver Transplantation. 2014;20:744–5. http://dx.doi.org/10.1002/lt.23853
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. http://dx.doi.org/10.1016/j.ab.2016.10.021
- Tsikas D, Duncan MW. Mass spectrometry and 3-nitrotyrosine: strategies, controversies, and our current perspective. Mass Spectrom Rev. 2014;33:237–76. http://dx.doi.org/10.1002/mas.21396
- Tsikas D. The dilemma of oxidative stress personified by the diprosopus 8-iso-prostaglandinf F2α and prostaglandin F2α. J Controvers Biomed Res. 2017;3:11–15. http://dx.doi.org/10.15586/jcbmr.2017.20
- Tsikas D, Suchy MT, Niemann J, Tossios P, Schneider Y, Rothmann S, et al. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F2α. FEBS Lett. 2012;586:3723–30. http://dx.doi.org/10.1016/j.febslet.2012.09.001
- Thum T, Batkai S, Malinski PG, Becker T, Mevius I, Klempnauer J, et al. Measurement and diagnostic use of hepatic cytochrome P450 metabolism of oleic acid in liver disease. Liver Int. 2010;30:1181–8. http://dx.doi.org/10.1111/j.1478-3231.2010.02310.x
- Tsikas D, Mitschke A, Gutzki FM, Meyer HH, Frölich JC. Gas chromatography mass spectrometry of cis-9,10-epoxyoctadecanoic acid (cis-EODA). II. Quantitative determination of cis-EpOA in human plasma by GC-tandem MS. J Chromatogr B. 2004;804:403–12. http://dx.doi.org/10.1016/j.jchromb.2004.01.055
- Trettin A, Böhmer A, Zoerner AA, Gutzki FM, Jordan J, Tsikas D. GC-MS/MS and LC-MS/MS studies on unlabelled and deuterium-labelled oleic acid (C18:1) reactions with peroxynitrite (O=N-O-O−) in buffer and hemolysate support the pM/nM-range of nitro-oleic acids in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:172–9. http://dx.doi.org/10.1016/j.jchromb.2014.01.016
- Vitetta L, Coulson S, Linnane AW. Reactive oxygen species in disease: Rebuttal of a conventional concept. J Controvers Biomed Res. 2015;1:23–27. http://dx.doi.org/10.15586/jcbmr.2015.7
- Woodford FP, Whitehead TP. Is measuring serum antioxidant capacity clinically useful? Ann Clin Biochem. 1998;35(Pt1):48–56. http://dx.doi.org/10.1177/000456329803500105
- Bartosz G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic Res. 2010;44:711–20. http://dx.doi.org/10.3109/10715761003758114
- de Vries DK, Kortekaas KA, Tsikas D, Wijermars LG, van Noorden CJ, Suchy MT, et al. Oxidative damage in clinical ischemia/reperfusion injury: A reappraisal. Antioxid Redox Signal. 2013;19:535–45. http://dx.doi.org/10.1089/ars.2012.4580
- Becker T, Mevius I, de Vries DK, Schaapherder AF, zu Vilsendorf AM, Klempnauer J, et al. The L-arginine/NO pathway in end-stage liver disease and during orthotopic liver and kidney transplantation: Biological and analytical ramifications. Nitric Oxide. 2009;20:61–7. http://dx.doi.org/10.1016/j.niox.2008.10.002
- Tsikas D, Schwedhelm E, Suchy MT, Niemann J, Gutzki FM, Erpenbeck VJ, et al. Divergence in urinary 8-iso-PGF(2alpha) (iPF(2alpha)-III, 15-F(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:237–55. http://dx.doi.org/10.1016/S1570-0232(03)00457-4
- Vascotto C, Cesaratto L, D’Ambrosio C, Scaloni A, Avellini C, Paron I, et al. Proteomic analysis of liver tissues subjected to early ischemia/reperfusion injury during human orthotopic liver transplantation. Proteomics. 2006;6:3455–65. http://dx.doi.org/10.1002/pmic.200500770
- Wu B, Wu H, Chen J, Hua X, Li N, Lu M. Comparative proteomic analysis of human donor tissues during orthotopic liver transplantation: Ischemia versus reperfusion. Hepatol Int. 2013;7:286–98. http://dx.doi.org/10.1007/s12072-012-9346-7
- Tsikas D, Hanff E, Becker T. Drastic decrease of global L-arginine bioavailability during orthotopic liver transplantation: A matter of ATP deficiency of the graft? Nitric Oxide. 2017. http://dx.doi.org/10.1016/j.niox.2017.02.009
- Tsikas D, Sawa M, Brunner G, Gutzki FM, Meyer HH, Frölich JC. Gas chromatography-mass spectrometry of cis-9,10-epoxyoctadecanoic acid (cis-EODA). I. Direct evidence for cis-EODA formation from oleic acid oxidation by liver microsomes and isolated hepatocytes. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:351–65. http://dx.doi.org/10.1016/S1570-0232(02)00821-8
- Tsikas D, Niemann J, Flentje M, Schwarz A, Tossios P. N-Acetylcysteine (NAC) inhibits renal nitrite and nitrate reabsorption in healthy subjects and in patients undergoing cardiac surgery: risk of nitric oxide (NO) bioavailability loss by NAC? Int J Cardiol. 2014;177:30–3. http://dx.doi.org/10.1016/j.ijcard.2014.09.109
- Burke A, FitzGerald GA, Lucey MR. A prospective analysis of oxidative stress and liver transplantation. Transplantation. 2002;74:217–21. http://dx.doi.org/10.1097/00007890-200207270-00012
- Galley HF, Richardson N, Howdle PD, Walker BE, Webster NR. Total antioxidant capacity and lipid peroxidation during liver transplantation. Clin Sci. 1995;89:329–32. http://dx.doi.org/10.1042/cs0890329
- Biasi F, Bosco M, Chiappino I, Chiarpotto E, Lanfranco G, Ottobrelli A, et al. Oxidative damage in human liver transplantation. Free Radic Biol Med. 1995;19:311–17. http://dx.doi.org/10.1016/0891-5849(95)00024-R
- Liu D, Luo G, Luo C, Wang T, Sun G, Hei Z. Changes in the concentrations of mediators of inflammation and oxidative stress in exhaled breath condensate during liver transplantation and their relations with postoperative ARDS. Respir Care. 2015;60:679–88. http://dx.doi.org/10.4187/respcare.03311
- Peel AM, Crossman-Barnes CJ, Tang J, Fowler SJ, Davies GA, Wilson AM, Loke YK. Biomarkers in adult asthma: A systematic review of 8-isoprostane in exhaled breath condensate. J Breath Res. 2017;11:016011. http://dx.doi.org/10.1088/1752-7163/aa5a8a
- van Beurden WJ, Harff GA, Dekhuijzen PN, van den Bosch MJ, Creemers JP, Smeenk FW. An efficient and reproducible method for measuring hydrogen peroxide in exhaled breath condensate. Respir Med. 2002;96:197–203. http://dx.doi.org/10.1053/rmed.2001.1240
- Duncan MW. Good mass spectrometry and its place in good science. J Mass Spectrom. 2012;47:795–809. Erratum In: J Mass Spectrom. 2012;47:1671. http://dx.doi.org/10.1002/jms.3038
- Tsikas D. What we-authors, reviewers and editors of scientific work-can learn from the analytical history of biological 3-nitrotyrosine. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1058:68–72. http://dx.doi.org/10.1016/j.jchromb.2017.05.012

