Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal, adult-onset, multifactorial, and progressively neurodegenerative disorder of motor neurons within the brainstem and spinal cord. Due to the progressive nature of the disease, symptoms worsen temporally as motor neurons deteriorate and muscle atrophy becomes increasingly established. Severe respiratory complications typically culminate in death 2-5 years following diagnosis. In North America, the incidence rate of ALS is approximately 2 per 100,000 and is expected to increase with an aging demographic (1). To date, more than 20 genetic mutations have been identified in familial forms of ALS (fALS); accounting for approximately 5% of all cases (2, 3). The most common underlying genetic causes of fALS pathogenesis include the recently characterized C9orf72 intronic hexanucleotide repeat expansion, as well as mutations in the genetic loci for the Cu/Zn superoxide dismutase 1 enzyme (SOD1), TAR DNA binding protein (TARDBP), and Fused in Sarcoma (FUS) RNA-binding protein (Figure 1; reviewed in 3, 4). Implicated in up to 20% of all fALS cases, more than 180 toxic-gain-of-function mutations in the SOD1 gene has been described throughout all exonic sequences (Figure 2; 3, 5). The vast majority of ALS patients (95%), however, suffer from a sporadic form of the disease (sALS) in which unknown etiological factors are causal in inducing the disease (4, 6).
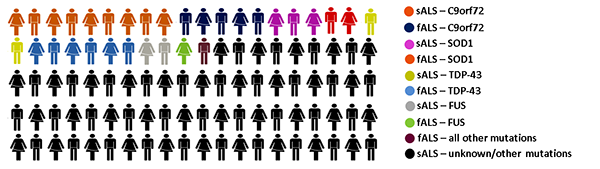
Figure 1. Graphical representation of the approximate frequencies of
familial and sporadic ALS cases in the overall affected patient population,
rounded to the nearest integer. Frequency of causes are expressed per 100 ALS
patients and do not account for sex differences. Infographic generated from the upper
frequency limit summarised in (3).
From
a clinical and pathological standpoint, both forms of ALS (SOD-1 fALS and sALS)
have primarily been considered to be virtually indistinguishable, since it is
thought that different initiating events ultimately converge to induce a
similar mechanistic cascade of disease progression (7, 8). This has led to the notion that if
researchers can dissect and understand the defective pathway(s) identified in
the context of genomic SOD1 abnormalities, that this knowledge can be
translated to sporadic forms of the disease and thus aid in therapeutic
development.
After more than two decades of work, various SOD1 genetic anomalies have been identified and actively studied in rodent models. An overarching pathogenic mechanism of action, however, remains elusive and the majority of therapeutic agents investigated have failed to yield positive outcomes when translated to the clinical level. Further challenging this reductionist viewpoint are neuropathological findings which suggest that the phenotypic expression of ALS may be consequent to heterogeneic pathologies affecting the central nervous system (CNS). For instance, by utilizing antibodies specific for labeling misfolded conformations of the SOD1 protein, some groups have demonstrated positive immunolabeling in fALS cases, a finding not always recapitulated in sALS (9–12). Similarly, pathological TDP-43 positive cytosolic inclusions common in sALS have not been described in mutant SOD1-linked fALS (13); a finding which is not always observed in mutant SOD1 models (14, 15). Taken together, these outcomes may signify diverse pathological cascades which ultimately present with the typical ALS phenotypic end state.
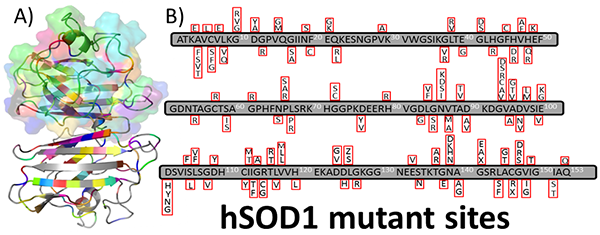
Figure 2. Distribution of hSOD1 amino acid substitutions within the monomer. A) PyMOL1 graphical visualization of the SOD1 protein identifying >150 amino acid substitutions throughout the 3D structure of the protein. B) The panel outlines the sites of substitution within the primary sequence of the polypeptide. Sites of the mutant residues depicted are derived from the ALS Online Genetic Database (5). 1The PyMOL Molecular Graphics System. Schrodinger, LLC.
Historically,
mutations within the SOD1 locus were first associated with the clinical
presentation of ALS, and were instrumental in developing the initial animal
models that have been employed in pre-clinical research (16–18). After more
than 50 randomized control trials testing various therapeutic agents which had
a positive effect in pre-clinical models, Riluzole remains the only
FDA-approved drug available to the affected patient population (19).
What
follows is a brief review of the common mutant SOD1 (mSOD1) fALS murine models,
the initial promising pre-clinical results, as well as the resultant outcomes
of select clinical trials. We will then shift our attention to a discussion
regarding the future applicability of these model systems in pre-clinical
research studies.
Murine models of familial
ALS
In the mid-nineties with the discovery that mutations in the antioxidant Cu/Zn-superoxide dismutase 1 (SOD1) gene locus was inheritable and linked to an ALS phenotype, there was renewed hope in the research community that a treatment was on the verge of being developed. To aid in therapeutic discovery, a murine model overexpressing a clinically-relevant mutant form of the human SOD1 enzyme with a glycine to alanine substitution at codon 93 (G93A) was created (17, 20–22). This result was soon followed by the development of mice overexpressing a variety of missense mutations in the SOD1 locus including G37R (18), D90A (23), G85R (24), and several other genomic abnormalities (25, 26). Due to the random genomic integration of the transgene, various lines of each mutation were established; each carrying the mutant gene to a varying degree and exhibiting a severity of disease that was highly dependent on the number of integrated transgenes (see Table 1 for the G37R and G93A mSOD1 models). These animal models were instrumental in delineating that the disease outcome was not a result of reduced dismutase activity, but due to a so-called “toxic-gain-of-function” property inherent to the mutant form of human SOD1 (22). More recent evidence, however, suggests that a loss of SOD1 activity may also contribute to disease presentation (27).
Table 1. Timing of the progressively degenerative phenotype in mSOD1
murine
models is highly dependent on the degree of mutant protein expression.
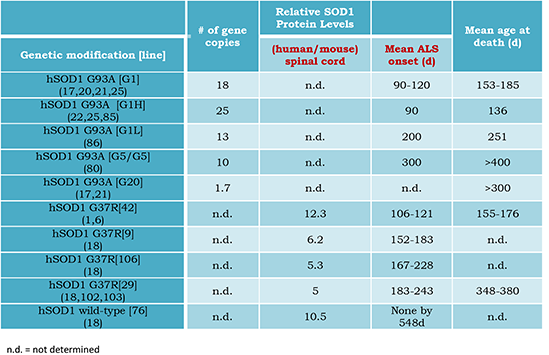
Prototypically,
the G93A mSOD1 animals experience a rapidly progressive phenotypic deterioration,
ultimately showing signs of hind limb paresis at approximately 150 days of age
(17). On account of the rapid and robust expression of the disease phenotype in
these animals, the G93A model has become the most widely used system in
assessing putative therapeutic agents (28). Neuropathologically this model
shows neuronal cell loss within the spinal cord as well as evidence for both
astrocytic and microglial proliferation; thus recapitulating some key features
inherent to clinical ALS cases (20, 29–32). Within this context, these in vivo mSOD1 models have provided a
glimpse into an aspect of the purported pathomechanism(s) underlying disease
presentation. This was thought to underscore their utility in not only studying
the inherent degenerative cascade, but also in translational therapeutic
development for all forms of ALS. However, since their inception, a plethora of
potential therapeutics have been tested in these models and similar positive
outcomes have not been recapitulated when applied to the clinical setting. The
paucity of positive clinical outcomes from therapies derived from pre-clinical
studies suggest that critically re-evaluating the sole reliance on mutant SOD1
models may identify non-productive research endeavours and accelerate therapeutic
development of efficacious agents.
Clinical applications
derived from investigations based on murine models
To
date, various therapeutics have been investigated for the ability to attenuate
and/or ameliorate the pathological disease cascade induced in ALS (19, 33).
Each of these agents addresses a unique hypothetical mechanism which is
purported to play a key role in establishing the eventual disease state.
Selected outcomes from both pre-clinical and clinical trials are discussed
below (also see Supplementary Tables S2, S3, and S4).
Riluzole
Glutamate
is a major excitatory neurotransmitter within the CNS (reviewed in 34). Once
released into the synaptic cleft, glutamate relays the action potential to the
downstream target neuron and the original signal is further propagated towards
the target site. Under normal physiological conditions, an intricate system of
neuronal and glial glutamate transporters result in the expedient endocytic
uptake of extracellular glutamate, and so remove the excitatory stimulus acting on the post-synaptic cell.
Increased levels of excess glutamate in the extracellular milieu will result
in excitotoxic activity of the post-synaptic cell due to aberrant Ca2+
homeostasis within the cytosol arising from excessive membrane depolarization
mediated through glutamate receptors (35). Increased cytosolic Ca2+
ions are compartmentalized within the mitochondrion, which can result in
alterations of mitochondrial permeability, production of reactive oxygen
species (ROS) and/or activating a plethora of enzymes involved in cell death
pathways (36, 37). Preliminary evidence invoking a role for glutamate in the
pathogenesis of ALS included an increase in peripheral levels (and a
concomitant decease in CNS tissue) in ALS patients (38, 39), reduced glutamate
uptake in patient-derived synaptosomes (40), and a reduction in levels of the
astrocytic glutamate transporter, EAAT2 (35). Riluzole, the only approved
pharmacological agent for treating ALS, has been shown to be effective in
extending survival, but only by a modest 2-3 months (41). A concrete mechanism
of action is yet to be elucidated; however, it appears to act on glutamate
transporters to facilitate the extracellular clearance of the excitotoxic
neurotransmitter (42), as well as inhibiting the presynaptic cell from
releasing glutamate (43). Synergistically, both of these actions would serve to
decrease the glutamate load in the extrasynaptic space and thus attenuate the proposed
cellular excitotoxic effects involved in disease pathogenesis.
In
contrast to the other ALS therapeutics discussed below, Riluzole treatment in
the clinical setting preceded validation in the ALS animal model, but was
explored based on evidence of anti-glutamatergic activity. The initial clinical
study at 100mg/day indicated a significant improvement in overall lifespan
after 12 months of treatment, a concomitant decrease in the rate of muscle
deterioration, and evidence of a more efficacious effect in patients exhibiting
bulbar-onset ALS (S3, S4; 44). Subsequent follow-up studies were shown to
replicate the initial therapeutic effect and investigated additional secondary
outcome measures. A dose-response study (50-200 mg/day) showed a significant improvement
in survival at higher doses. However Riluzole treatment failed to significantly
affect functional assessments (i.e. muscle testing, respiratory function, and
subjective symptom assessment), or differentially affect limb- or bulbar-onset
cases (45, 46). Population-based studies confirmed a positive therapeutic
effect of Riluzole treatment that particularly affected bulbar-onset patients.
However, the overall effect in ALS patients was transient in that the survival
curves were unaffected when examined in prolonged follow up studies (47, 48).
In ALS animal models (S2), Riluzole treatment
(100 micrograms/mL water) during the presymptomatic stage was initially shown to exert
no effect on delaying the onset of disease, but did significantly extend
survival by 13-15 days (49). A follow-up study showed a dose-dependent
preservation of motor function, while exhibiting an identical positive effect
of survival at doses of 24-44 mg riluzole/kg body weight/day of 12-13 days
(50). The positive, but modest, clinical effect combined with an extended
survival in the fALS model has solidified the notion of the clinical utility of
Riluzole as a therapeutic agent in ALS, which to date, remains the only drug
approved for treatment.
Minocycline
Minocycline
is a lipid-soluble, semi-synthetic tetracycline antibiotic that can readily
penetrate the blood-brain barrier, and has been shown to exhibit
anti-inflammatory properties apart from its expected antimicrobial activity
(51). Initial work in models of neurodegeneration had shown that treatment with
the antibiotic prevented microglial activation, inhibited release of the
pro-inflammatory cytokine IL-1β, and rescued primary neuronal cells from
glutamate cytotoxicity (51, 52). Increased neuroinflammatory processes and the
activation of microglia have long been suspected in the underlying
pathomechanism of ALS (53). The strong preliminary data in models of
neurodegeneration warranted further study in the context of ALS. Mutant SOD1
animal models (G37R or G93A) receiving minocycline either in their diet or via
intraperitoneal injections during the presymptomatic phase of the disease,
showed evidence of a dose-dependent delay in onset and an attenuated
progression of disease (S2; 54–56).
Following preliminary studies assessing the safety and tolerability of
minocycline in ALS patients (57, 58), a randomised phase 3 clinical trial set
out to test the efficacy of the treatment paradigm (S3 and 4; 59). In contrast to pre-clinical findings, oral dosage of
minocycline was found to be associated with a significantly more rapid decline
in functional outcome scores (as measured by the revised ALS functional rating
scale), while concurrently showing evidence for a reduction in lung capacity
and muscle performance. Although pre-clinical evidence showed a marked
attenuation of disease presentation, similar findings were not recapitulated
within the clinical setting, and treatment with minocycline seemed to
exacerbate ALS-related deterioration.
Creatine
Mitochondria
are crucial in generating the intracellular energy stores required to drive
biochemical processes, maintain calcium homeostasis, and play a role in
regulating apoptosis (60). Murine models of SOD1-ALS show evidence of aberrant
mitochondrial pathology; most notably mitochondrial swelling and vacuolization
(18, 20). Functionally, in vivo model
systems expressing the G93A missense mutation in the SOD1 locus showed evidence
for a decrease in mitochondrial membrane potential, an increase in the
concentration of cytosolic calcium ions, as well as an increased susceptibility
to oxidative stress (61, 62). Clinically, ALS patients show aberrant
mitochondrial pathology in that a dense aggregation of mitochondria is
localized to the presynaptic terminal (63). Such dysfunction of mitochondria
would ultimately perturb the energy balance of the system and contribute to
cellular degeneration. Therapeutics directed at enhancing and/or stabilizing
normal mitochondrial functioning was thus speculated to be of some utility in
treating ALS. Creatine, generated through a biosynthetic pathway involving
arginine and glycine, exists in non-phosphorylated and phosphorylated forms.
Together, these biomolecules serve an important function in producing ATP at
sites of high energy consumption (64, 65). Exogenously administered creatine
was shown to exert neuroprotective properties through various inter-related
pathways including attenuating glutamate-induced cytotoxicity (66), and
directly acting as an antioxidant against reactive ion species (67). Elevated
creatine levels are thought to protect mitochondrial creatine kinase from
oxidative damage which inhibits the opening of the mitochondrial transition
pore, and so protects against cell death (68, 69). Oral creatine treatment in
the G93A model showed evidence of a dose-dependent increase in overall lifespan
(26 days at 2% w/w creatine supplementation compared totransgenic controls),
improved motor performance, and exhibited an increase in neuronal viability
comparable to wild-type littermates in a cohort receiving 1% dietary creatine (S2; 70). In the context of a lower copy
number, dietary creatine (2% w/w) was shown to delay symptom onset by 12 days
and decrease the severity of disease presentation, however, this did not
correlate with a significant amelioration of motor neuron loss (71).
Combinatorial approaches showed that creatine dosage supplemented with
minocycline, but not Riluzole, resulted in an additive therapeutic effect with
a delay in phenotypic onset and a concomitant increase in overall lifespan (71,
72). Clinically at a dose of 10g/day, dietary creatine intake (for 16 months)
did not positively affect survival rates or the degree of decline for a variety
of clinically-relevant measures (65). A
concurrent study at a lower dosage (5g/day) for six months examining a different
set of outcome parameters similarly failed to establish a role for creatine in
ameliorating ALS pathogenesis (73). Following a preliminary study with positive
outcomes, Rosenfeld et al. set out with a larger cohort and followed patients
for nine months receiving creatine at 5g/day (74). As in the previous clinical trials, dietary
supplementation failed to demonstrate a therapeutic benefit in multiple
measures of relevance to the clinical presentation of ALS (S3 and S4). The lack of
positive correlation between pre-clinical models and clinical trials in
therapeutic outcomes of creatine treatment has halted further clinical
investigation of the agent.
Limitations of clinical
ALS trials and modeling disease in mouse models expressing mutant human SOD1
Having
briefly reviewed the clinical outcomes of three selected therapeutic agents, it
is likely that the discrepancy between pre-clinical results and clinical
translation are multifactorial in nature. Mounting evidence supports a
heterogeneous cascade of events that underlie the inherent pathomechanism(s) of
ALS (6). Clinically, patients present with a phenotypically heterogeneous
disease that can affect site of onset, rate of disease progression, upper
and/or lower motor neuron involvement, and whether the phenotype is strictly
behavioural or elicits some form of cognitive impairment (75). It is probable
that inadequate stratification of the patient population in terms of phenotypic
variability (and perhaps yet unknown genetic and environmental causal factors)
could undermine robust clinical outcomes, since any specific treatment effects
may only be applicable to a subset of ALS patients (19, 76).
The
theoretical utility of animal models is in their ability to mimic the
underlying disease cascade and thus act as a platform for therapeutic
development. A model system should be representative of the patient population
as a whole so that any pre-clinical drug development should be applicable when
translated clinically. In the case of ALS, there is roughly a global prevalence
rate of 4-7 per 100, 000 population; thus around half a million patients would
be affected world-wide (1). Familial ALS cases due to mutant SOD1 loci would
then account for approximately 5000-10,000 patients. Pre-clinical development
based solely on a model of mutant SOD1 thus has the potential to greatly impede
translational applicability since (i) such a small percentage of patients that
comprise the clinical trial will be carrying a mutant copy of the SOD1 gene,
and (ii) logistically enrolling SOD1-affected fALS patients in a trial of
sufficient power to properly test a developed therapy would prove daunting.
Further,
as is evidenced in this brief review, there is limited proof for translational
applicability of therapeutics developed in mSOD1 models of ALS: a caveat not
restricted to neurodegenerative disease research. Generally a positive
pre-clinical effect has less than a 40% chance of recapitulating a similar
clinical outcome (77), with some animal models not even accurately portraying
the inherent disease pathomechanism(s) (78).
Limited
independent validation studies, publication bias and deficits in good
experimental design may also account for some of the discrepancy in outcomes
between clinical and pre-clinical mSOD1 studies
(28). Furthermore, as Scott et al. poignantly discuss, failing to control for
specified biological factors in ALS models has likely resulted in the
measurement of chance variability (i.e.
“noise”), and has thus resulted in false positive results (79). To underscore
the importance of these variables, an optimized study paradigm investigating the
efficacy of Riluzole, minocycline, or creatine were unable to replicate the
positive pre-clinical outcomes previously discussed (79).
In
short, due to the complex interplay of etiological factors, effectively
modeling ALS has proven to be quite challenging. Thus, the discrepancy in
translating therapeutics clinically is likely multifactorial in nature and will
be the focus of the remaining discussion. Of primary concern here is how well
the in vivo model mimics clinical
ALS, impediments hindering replicability between pre-clinical studies, as well
as the importance of species differences in drug metabolism.
Models representative of
clinical ALS
Focusing
on the original line of G93A mSOD1 animals with the greatest number of
transgene integrations (G1), neuropathological changes are of a progressively
degenerative nature (17, 20, 21). At the outset, vacuolar degeneration is
observed within motor neurons, but with time, alongside a marked reduction of
neuronal cell bodies, pathological vacuoles are present within the surrounding
neuropil with a marked deterioration of the anterior horn. Evidence for
vacuolar degeneration in animals highly expressing the mutant G93A SOD1 locus
does not reflect the reality of end-state pathology in human cases, and has
been suggested to be a toxic artefact arising from the transgene being
significantly overexpressed (21). Thus, it is possible that the marked
overexpression of the mutant transgene results in a more severe disease
pathology which may not reliably measure a positive or negative clinical
outcome for translational purposes. Within the context of an accelerated
disease cascade, it is probable that failed therapeutic agents at the
pre-clinical stage may have had some clinical utility in the protracted form of
human disease. However, without strong pre-clinical data in these progressive
models, candidate therapeutics will not be promoted to the clinical trial
stage.
Expression
of the aberrant phenotype in SOD1 fALS models are driven by the significant
overexpression of the mutant hSOD1 locus (Table
1). In stark contrast, familial forms of the disease (SOD1-linked fALS) are
inherited mostly in an autosomal dominant manner and thus present with ALS in
the context of one aberrant copy. Transgenic lines carrying significantly fewer
copies of various gain-of-function mutations do not develop disease after
prolonged observation (17, 18), or give rise to a significantly protracted
disease course (80). For instance the SOD1A4V strain of mice carry
the most common SOD1 mutation found in North America (81), yet fail to
recapitulate the requisite phenotypic correlates of disease (17). This is
primarily thought to be a consequence of decreased transgene expression levels.
Of the two A4V lines developed, the line expressing the mutant protein to a
higher degree (20% greater than the lower expressing line) was shown to exhibit
an affected phenotype only when crossbred with a line overexpressing wild-type
hSOD1 suggesting that a threshold level of mutant SOD1 needs to be exceeded
prior to phenotypic onset (82).
An
additional impediment in utilizing the model to predict clinical efficacy is in
the timing of drug administration. Typically the timing of drug delivery in
these models is prior to phenotypic onset and thus provides a wide therapeutic
window for the effect to be realized (S2).
In lieu of biomarkers which specifically gauge the onset and progression of the
disease, a typical therapeutic intervention initiated in the clinic will only
be administered following symptom onset; most notably at a stage of the disease
when marked neuronal cell loss has already occurred. Due to the intractable and
rapidly progressive nature of ALS, it should not be surprising that many
promising pre-clinical therapeutics have not been able to attenuate and/or
ameliorate the disease when initiated at an advanced stage when translated to
the clinic.
Impediments to
replicability between pre-clinical studies
The
literature is rife with studies exemplifying the relationship between mutant
hSOD1 transgene levels incorporated into the genome and the degree of
phenotypic severity. Our recent experience with the G37R model underscores this
dilemma in that we had generated a colony of animals from commercial breeders
with an uncharacterized drop in copy number. As expected, these presented with
a concomitant increase in lifespan and delay in disease progression (83).
Transgene level variation can arise due to meiotic recombination. In the G93A
mSOD1 model, this is relatively infrequent with recombination accounting for
transgene fluctuations in approximately 3% of the progeny produced (84). Due to
random meiotic events, the original G93A G1 line has spawned two additional
sub-lines with either a 40% expansion (25 copies) or 30% retraction (13 copies)
of the mutant hSOD1 locus; each showing variations in phenotype severity on
account of altered transgene expression levels (85, 86).
Experimentally,
variations in transgene dosage are detrimental to research efforts. First,
small colonies are likely to be adversely impacted and would serve limited
applicability in replication studies. Second, undetected fluctuations in
individual copy number will give rise to animals exhibiting varied phenotypic
expression of disease severity, and thus respond differently to therapeutic
intervention. Should an experimental cohort by chance be comprised of outliers
with either higher/lower copy numbers, a tested therapeutic effect may be
primarily a consequence of disease severity linked to transgene levels. Without
dutifully reporting on the number of integrated transgenes/mSOD1 expression
levels, the issue is further compounded when independent groups attempt to
replicate previous results.
The
incongruent findings between clinical and pre-clinical studies may further be
explained by underlying physiological differences in metabolizing the
therapeutic. To the authors’ knowledge, no investigation has yet been conducted
to study whether any putative ALS pharmacological agent is processed similarly
in both mice and man, and thus whether it is bioactive to the same degree in
these different species. Although it is used to induce a form of parkinsomism,
systemic administration of the neurotoxic agent MPTP exemplifies
species-specific differences in metabolic activity (87). In humans and primate
models, intravenous delivery mediates an acute parkinsonian syndrome virtually
indistinguishable from idiopathic Parkinson’s disease. Rodent models however
have proven to be somewhat more resilient to the toxic insult accompanying
systemic administration. In rats, because of peripheral enzymatic catabolism of
MPTP by monoamine oxidase, the polar MPP+ metabolite cannot cross the
blood-brain-barrier and thus fails to selectively induce dopaminergic neuron
degeneration (87). Without dutifully assessing the possibility of cross-species
drug metabolic differences, it is possible that any pre-clinical effect may not
be fully recapitulated in the clinic.
Future considerations for
modeling disease and testing clinically-relevant ALS therapeutics
Due
to the definitive need for therapeutics targeting the neurodegenerative
cascade(s) inherent to ALS pathogenesis, it is of crucial importance that
non-productive research endeavours are expediently identified and resources
redirected to more promising avenues. To date, the reductionist approach of
understanding ALS as a mSOD1-mediated insult has not borne out an effective
treatment strategy as was initially expected. In contrast to the current
approach of studying potential therapeutics in the context of a specific
genetic causality, a disease such as ALS with a complex etiology necessitates a
multi-pronged strategy. Herein we broadly consider future strategies that could
greatly facilitate the development and testing of potential ALS therapies.
Clinically-relevant
biomarkers: assessing disease progression and therapeutic efficacy
There
is a desperate need for the development of clinically-relevant biomarkers that
will not only aid in an earlier diagnosis of disease, but also provide
information with regard to disease progression. To date, various potential
peripheral blood biomarkers have been identified, but none have been
successfully translated into clinical use (reviewed in 88). Identification of
such biomarkers would not only aid in diagnosing patients at an earlier stage
of the disease, but also provide for an alternate measure of the effectiveness
of any putative therapeutic agents.
Having
a distinct correlative marker of disease progression might also shorten
clinical trials since the effectiveness of a drug could be more efficiently assessed
(88, 89). With shorter clinical trials, patients could then participate in more
studies, thus allowing for more therapeutics to be clinically assessed and a
treatment regimen to be fast-tracked. In addition, predictive biomarkers would
allow for an earlier diagnosis of disease. This could facilitate an earlier
intervention strategy in the progressive neurodegenerative cascade and
theoretically mitigate additional CNS damage.
Blood
provides for an easily-obtained biological fluid which constitutes a viable
source for biomarker discovery, with the added caveat that blood biomarkers may
not directly correlate with motor neuron degeneration (88). As Robelin and De
Aguilar discuss, both the blood-brain barrier and the blood-cerebrospinal fluid
barrier may act as an impediment to the crossing of relevant biomarkers into
systemic circulation (88). There is thus a possibility that surrogate
peripheral biomarkers may not adequately reflect the underlying degenerative
cascade, or provide a direct measure of the intended therapeutic effect within
the CNS. On account of the complex neurodegenerative cascade at play during ALS
pathogenesis, it may be more appropriate to identify panels of biomarkers that
clearly distinguish ALS from related disorders (88).
A
recently conducted clinical trial of rasagiline, an inhibitor of monoamine
oxidase B, demonstrates the application of potential blood biomarkers in
assessing the clinical outcome of a therapeutic agent (90). Prior work had
demonstrated that the drug had specific beneficial effects on mitochondrial
function, decreased oxidative damage, and inhibited apoptosis. As previously
pointed out, mitochondrial dysfunction is a clinical hallmark of ALS
pathogenesis. Thus measuring particular biomarkers related to mitochondrial
function (e.g. changes in mitochondrial membrane potential, oxidative stress,
and the relative abundance of pro-survival/pro-apoptotic signals) may allow for
a biochemically-relevant measure of therapeutic effectiveness in ALS clinical
trials (90). The trial did not demonstrate an improvement in the functional
ALSFRS-R scores after 12 months compared to historical controls (when corrected
for symptom duration). However, peripheral lymphocytes showed evidence of an
increased mitochondrial membrane potential, decreased oxidative stress, and an
increased Bcl-2: Bax ratio; indicative of pro-survival cell signaling. A major
caveat to the use of peripheral biomarkers as a surrogate indicator for
activity within the CNS is the degree to which the therapeutic agent is able to
penetrate, and act on cells within the CNS. In the case of rasagiline, the use
of a peripheral mitochondrial biomarker is expected to be an adequate indicator
of CNS activity since the therapeutic agent has demonstrated peripheral
distribution and CNS penetration (90).
Development of alternate
models to more effectively mirror the diverse etiological factors that underlie
ALS pathogenesis
A
critical problem lies in developing animal models which are more representative
of ALS patients. To date, primarily on account of the unknown etiology, there
is a lack of models that are representative of the sporadic form of the
disease. Our group has previously investigated the dietary administration of
cycad flour or extracted sterol glucosides as causative agents in the purported
disease cascade (91-93), while others have assessed low Calcium/Magnesium, high
Aluminum diets (94). Potential therapeutics have yet to be formally
investigated within the context of these environmental model systems.
Furthermore, there needs to be a renewed effort to develop and validate
additional animal models with suspected environmental etiological factors.
On
account of our increased understanding of the underlying genetic causal factors
at play in ALS (Figure 1), there
have been multiple additional murine models developed since the advent of the
mutant SOD1 mouse. These include mice expressing mutant TDP-43 or the
C9ORF72-associated hexanucleotide repeat expansions (95-97). Characterising
these additional systems allow for a more representative model of the overall
ALS population as it takes into account the multiple underlying pathological
changes that are implicated in disease pathogenesis.
However,
a central caveat to conducting pre-clinical studies in animal models is the
inability to rapidly screen multiple therapeutic agents in a single assay. To
address this, an approach involves modeling the disease with nerve cells
derived from induced pluripotent stem cells from sALS and/or fALS patients (98,
99). Not only could this strategy allow for the screening of a plethora of
neuroprotective compounds, but it may pave the way for determining
patient-specific drug responses to ALS therapeutics. An alternate approach
employs the use of small invertebrate and vertebrate model systems expressing
mutant forms of loci associated with ALS as platforms for pre-clinical drug
development. Zebrafish and Caenorhabditis elegans animal models
that express mutant forms of human TDP-43 or FUS allow for the production of
large numbers of animals that can be housed in multi-well plates, tested with
an array of potential therapeutic compounds, and assessed whether the disease
phenotype is attenuated (100). Strategies such as these may allow for the rapid
identification of potential therapeutic agents that can ultimately be applied
at the clinical level.
Realistically,
due to the heterogeneity of the pathological mechanism(s) at play, it is quite
unlikely that any one model would be sufficient in mimicking all of the
relevant underlying pathobiology. What should be adopted instead is a
multi-pronged approach where a potential therapeutic is validated in multiple
model systems testing specific outcomes prior to clinical translation. Adopting
this strategy would enhance the likelihood of success since a treatment with
positive outcomes in multiple model systems will be of greater relevance to the
heterogeneous patient population.
Clinical trials should be
designed and stratified so that the therapeutic effect of any agent is tested
in a patient subset that is homologous for set criteria
Clinical
ALS trials should be conducted in a manner that reflects the pathobiological
heterogeneity underlying the neurodegenerative cascade. As discussed elsewhere,
current evidence suggests that the disease presents along a continuum which
ranges from “pure” ALS to frontotemporal dementia (6, 101). Employing a
heterogeneous study population in a clinical trial with undefined disease
mechanisms will not see positive outcomes unless all disease pathways converge
for the ultimate phenotypic expression. It is possible that this may not be the
case in ALS. That said, stratifying patient cohorts based on both the
underlying causal mechanism(s) and degree of disease progression (i.e. due to
validated biomarkers), would allow clinical researchers to develop targeted
strategies that address definite causal mechanisms within a specific context.
Conclusion
We
have reviewed that the most commonly used animal model in pre-clinical ALS
therapeutic development is only representative of a small proportion of
clinical cases, and has been shown to be a poor predictor of clinical success.
To date, Riluzole is the only FDA-approved therapeutic, but one which has only
a modest effect on overall lifespan and disease progression. All other
therapeutics with positive pre-clinical outcomes in the murine model have not
successfully translated to the clinical setting. The reasons for this are
multifaceted in nature and not limited to a publication bias against negative
pre-clinical data, a lack of reproducibility between research groups, a
reductionist paradigm in pre-clinical testing, and a patient population
affected by potentially multiple causal factors. As our understanding of the
complex etiological factors in ALS evolves over the coming years, we can
investigate multiple therapeutic compounds acting on diverse disease mechanisms
and perhaps more effectively intervene in a large proportion of the affected
populace.
Conflict
of Interest
CAS
is the Co-founder of Neurodyn Inc.
Acknowledgement
The
authors thank the Luther Allyn Shrouds Dean Estate for their support.
References
1.
Chio A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, White
LA. Global epidemiology of amyotrophic lateral sclerosis: a systematic review
of the published literature. Neuroepidemiology 2013; 41(2): 118–130.
http://dx.doi.org/10.1159/000351153
2.
Byrne S, Walsh C, Lynch C, Bede P, Elamin M, Kenna K, McLaughlin R, Hardiman O.
Rate of familial amyotrophic lateral sclerosis: a systematic review and
meta-analysis. J Neurol Neurosurg Psychiatry 2011; 82: 623–7.
http://dx.doi.org/10.1136/jnnp.2010.224501
3.
Harms M, Baloh R. Clinical Neurogenetics. Neurol Clin 2013; 31: 929-950.
http://dx.doi.org/10.1016/j.ncl.2013.05.003
4.
Marangi G, Traynor BJ. Genetic causes of amyotrophic lateral sclerosis: new
genetic analysis methodologies entailing new opportunities and challenges.
Brain Res 2015; 1607: 75–93.
http://dx.doi.org/10.1016/j.brainres.2014.10.009
5.
Amyotrophic Lateral Sclerosis Online Genetic Database (ALSOD).
http://alsod.iop.kcl.ac.uk/Overview/gene.aspx?gene_id=SOD1
6.
Turner MR et al. Controversies and priorities in amyotrophic lateral sclerosis.
Lancet Neurol 2013; 12: 310–322.
http://dx.doi.org/10.1016/S1474-4422(13)70036-X
7.
Kabashi E, Valdmanis PN, Dion P, Rouleau GA. Oxidized/misfolded superoxide
dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann Neurol 2007;
62: 553–559.
http://dx.doi.org/10.1002/ana.21319
8.
Bowling AC, Schulz JB, Brown RH. Superoxide dismutase activity, oxidative
damage, and mitochondrial energy metabolism in familial and sporadic
amyotrophic lateral sclerosis. J. Neurochem 1993; 61(6): 2322-2325.
http://dx.doi.org/10.1111/j.1471-4159.1993.tb07478.x
9.
Liu H-N, Sanelli T, Horne P, Pioro E, Strong M, Rogaeva E, Bilbao J, Zinman L,
Robertson J. Lack of evidence of monomer/misfolded superoxide dismutase-1 in
sporadic amyotrophic lateral sclerosis. Ann Neurol 2009; 66: 75–80.
http://dx.doi.org/10.1002/ana.21704
10.
Ayers J, Xu G, Pletnikova O, Troncoso J, Hart J, Borchelt D. Conformational
specificity of the C4F6 SOD1 antibody; low frequency of reactivity in sporadic
ALS cases. Acta Neuropathol Commun 2014; 2(55): 1-13.
http://dx.doi.org/10.1186/2051-5960-2-55
11.
Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant
localization of FUS and TDP43 is associated with misfolding of SOD1 in
amyotrophic lateral sclerosis. PLoS ONE 2012; 7(4): 1-9.
http://dx.doi.org/10.1371/journal.pone.0035050 PMid:22493728
PMCid:PMC3320864.
12.
Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic
ALS pathogenesis. Front Cell Neurosci 2013; 7: 1-16.
http://dx.doi.org/10.3389/fncel.2013.00253
13.
Mackenzie I et al. Pathological TDP-43 distinguishes sporadic amyotrophic
lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann
Neurol 2007; 61: 427–434.
http://dx.doi.org/10.1002/ana.21147
14.
Shan X, Vocadlo D, Krieger C. Mislocalization of TDP-43 in the G93A mutant SOD1
transgenic mouse model of ALS. Neurosci Lett 2009; 458: 70–74.
http://dx.doi.org/10.1016/j.neulet.2009.04.031
15.
Turner BJ, Baumer D, Parkinson NJ, Scaber J, Ansorge O, Talbot K. TDP-43
expression in mouse models of amyotrophic lateral sclerosis and spinal muscular
atrophy. BMC Neurosci 2008; 9(104): 1-11.
http://dx.doi.org/10.1186/1471-2202-9-104
16.
Rosen D et al. Mutations in Cu/Zn superoxide dismutase gene are associated with
familial amyotrophic lateral sclerosis. Nature 1993; 362: 59–62.
http://dx.doi.org/10.1038/362059a0
17.
Gurney M, Pu H, Chiu A, Canto M, Polchow C, Alexander D, Caliendo J, Hentati A,
Kwon Y, Deng H. Motor neuron degeneration in mice that express a human Cu,Zn
superoxide dismutase mutation. Science 1994; 264: 1772–1775.
http://dx.doi.org/10.1126/science.8209258
18.
Wong P, Pardo C, Borchelt D, Lee M, Copeland N, Jenkins N, Sisodia S, Cleveland
D, Price D. An adverse property of a familial ALS-linked SOD1 mutation causes
motor neuron disease characterized by vacuolar degeneration of mitochondria.
Neuron 1995; 14: 1105-1116.
http://dx.doi.org/10.1016/0896-6273(95)90259-7
19.
Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral
sclerosis: why so many negative trials andhow can trials be improved? Lancet
Neurol 2014; 13: 1127–1138.
http://dx.doi.org/10.1016/S1474-4422(14)70129-2
20.
Dal Canto D, Gurney ME. Development of central nervous system pathology in a
murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol
1994; 145(6): 1271–1279.
PMid:7992831
PMCid:PMC1887498.
21.
Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice
carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing
wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS).
Brain Res 1995; 676: 25–40.
http://dx.doi.org/10.1016/0006-8993(95)00063-V
22.
Gurney ME. The use of transgenic mouse models of amyotrophic lateral sclerosis
in preclinical drug studies. J Neurol Sci 1997; 152(Suppl. 1): S67-S73.
23.
Jonsson PA, Graffmo KS, Brannstrom T, Nilsson P, Andersen PM, Marklund SL.
Motor neuron disease in mice expressing the wild type-like D90A mutant
superoxide dismutase-1. J Neuropathol Exp Neurol 2006; 65(12): 1126–1136.
http://dx.doi.org/10.1097/01.jnen.0000248545.36046.3c
24.
Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS,
Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-Linked SOD1 Mutant G85R
Mediates Damage to Astrocytes and Promotes Rapidly Progressive Disease with
SOD1-Containing Inclusions. Neuron 1997; 18: 327-338.
http://dx.doi.org/10.1016/S0896-6273(00)80272-X
25.
Kato S. Amyotrophic lateral sclerosis models and human neuropathology:
similarities and differences. Acta Neuropathol 2007; 115: 97-114.
http://dx.doi.org/10.1007/s00401-007-0308-4
26.
Van Den Bosch L. Genetic Rodent Models of Amyotrophic Lateral Sclerosis. J
Biomed Biotechnol 2011; 1-11.
http://dx.doi.org/10.1155/2011/348765 PMid:21274268
PMCid:PMC3022221.
27.
Saccon RA, Bunton-Stasyshyn RKA, Fisher EMC, Fratta P. Is SOD1 loss of function
involved in amyotrophic lateral sclerosis? Brain 2013; 136: 2342–2358.
http://dx.doi.org/10.1093/brain/awt097 PMid:23687121
PMCid:PMC3722346.
28.
Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human
ALS. Neurobiol Dis 2007; 26: 1–13.
http://dx.doi.org/10.1016/j.nbd.2006.12.015
29.
Hall ED, Oostveen JOA, Gurney ME. Relationship of microglial and astrocytic
activation to disease onset and progression in a transgenic model of familial
ALS. Glia 1998; 23: 249–256.
http://dx.doi.org/10.1002/(SICI)1098-1136(199807)23:3<249::AID-GLIA7>3.0.CO;2-#
30.
Lewis KE, Rasmussen AL, Bennett W, King A. Microglia and motor neurons during
disease progression in the SOD1G93A mouse model of amyotrophic lateral
sclerosis:changes in arginase1 and inducible nitric oxide synthase. J
Neuroinflamm 2014; 11: 55
http://dx.doi.org/10.1186/1742-2094-11-55
31.
Boillee S, VandeVelde C, Cleveland DW. ALS: A Disease of motor neurons and
their nonneuronal neighbors. Neuron 2006; 52: 39-59.
http://dx.doi.org/10.1016/j.neuron.2006.09.018
32.
Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of
familial ALS correlates with disease progression. Neurology 2001; 57:
1282–1289.
http://dx.doi.org/10.1212/WNL.57.7.1282
33.
Wilkins HM, Bouchard RJ, Lorenzon NM, Linseman DA. Poor correlation between
drug efficacies in the mutant SOD1 mouse model versus clinical trials of ALS
necessitates the development of novel animal models for sporadic motor neuron
disease. In: Costa A, and Villalba E, eds. Horizons in Neuroscience Research
Vol 5. Nova Science Publishers, New York: 2011; 1-39.
34.
Danbolt NC. Glutamate uptake. Prog Neurobiol 2001; 65: 1–105.
http://dx.doi.org/10.1016/S0301-0082(00)00067-8
35.
Julien J-P. Amyotrophic Lateral Sclerosis: Unfolding the Toxicity of the
Misfolded. Cell 2001; 104: 581-591.
http://dx.doi.org/10.1016/S0092-8674(01)00244-6
36.
Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective
from the cell death community. Cell Calcium 2011; 50: 211–221.
http://dx.doi.org/10.1016/j.ceca.2011.03.003
37.
Jaiswal M, Zech W-D, Goos M, Leutbecher C, Ferri A, Zippelius A, Carri M, Nau
R, Keller B. Impairment of mitochondrial calcium handling in a mtSOD1 cell
culture model of motoneuron disease. BMC Neurosci 2009; 10: 64.
http://dx.doi.org/10.1186/1471-2202-10-64
38.
Plaitakis A, Constantakakis E. Altered metabolism of excitatory amino acids,
N-acetyl-aspartate and N-acetyl-aspartyl-glutamate in amyotrophic lateral
sclerosis. Brain Res Bull 1993; 30: 381–386.
39.
Camu W, Billiard M, Baldy-Moulinier M. Fasting plasma and CSF amino acid levels
in amyotrophic lateral sclerosis: a subtype analysis. Acta Neurol Scand 1993;
88: 51–55.
http://dx.doi.org/10.1111/j.1600-0404.1993.tb04186.x
40.
Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain
and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 1992; 326(22):
1464-1468.
http://dx.doi.org/10.1056/NEJM199205283262204
41.
Miller RG, JD M, Moore DH. Riluzole for amyotrophic lateral sclerosis
(ALS)/motor neuron disease (MND). Cochrane Database of Syst Rev 2012; CD001447.
PMid:22419278
42.
Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake
in rat spinal cord synaptosomes. Brain Res 2000; 871: 175–180.
http://dx.doi.org/10.1016/S0006-8993(00)02430-6
43.
Wang S-J, Wang K-Y, Wang W-C. Mechanisms underlying the riluzole inhibition of
glutamate release from rat cerebral cortex nerve terminals (synaptosomes).
Neurosci 2004; 125: 191–201.
http://dx.doi.org/10.1016/j.neuroscience.2004.01.019
44.
Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in
amyotrophic lateral sclerosis. N Engl J Med 1994: 330(9): 585-591.
http://dx.doi.org/10.1056/NEJM199403033300901
45.
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Powe L, Durrleman S, Delumeau JC,
Meininger V. A confirmatory dose-ranging study of riluzole in ALS. Neurology
1996; 47 (Suppl 4): S242–S250.
http://dx.doi.org/10.1212/WNL.47.6_Suppl_4.242S
46.
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of
riluzole in amyotrophic lateral sclerosis. Lancet 1996; 347: 1425–1431.
http://dx.doi.org/10.1016/S0140-6736(96)91680-3
47.
Traynor BJ, Alexander M, Corr B, Frost E, Hardiman, O. An outcome study of
riluzole in amyotrophic lateral sclerosis. J Neurol 2003; 250: 473-479.
http://dx.doi.org/10.1007/s00415-003-1026-z
48. Zoccolella S, Beghi E, Palagano G.
Riluzole and amyotrophic lateral sclerosis survival: a population‐based study in
southern Italy. Eur J Neurol 2007; 14: 262-268. http://dx.doi.org/10.1111/j.1468-1331.2006.01575.x
PMid:17355545.
49.
Gurney ME, Cutting FB, Zhai P, Doble A, Taylor, CP, Andrus PK, Hall ED. Benefit
of vitamin E, riluzole, and gababapentin in a transgenic model of familial
amyotrophic lateral sclerosis. Ann Neurol 1996; 39(2): 147-157.
http://dx.doi.org/10.1002/ana.410390203
50.
Gurney ME, Fleck TJ, Himes CS, Hall ED. Riluzole preserves motor function in a
transgenic model of familial amyotrophic lateral sclerosis. Neurol 1998; 50:
62–66.
http://dx.doi.org/10.1212/WNL.50.1.62
51.
Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines
inhibit microglial activation and are neuroprotective in global brain ischemia.
Proc Natl Acad Sci 1998; 95:15769-15774.
http://dx.doi.org/10.1073/pnas.95.26.15769
52.
Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A
tetracycline derivative, minocycline, reduces inflammation and protects against
focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci
1999; 96(23): 13496-13500.
53.
Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: the good, the bad, and
the resting. J Neuroimmune Pharmacol 2009; 4: 389–398.
http://dx.doi.org/10.1007/s11481-009-9171-5
54.
Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse
model of amyotrophic lateral sclerosis. Neurobiol Dis 2002; 10: 268-278.
http://dx.doi.org/10.1006/nbdi.2002.0487
55.
Van Den Bosch L, Tilkin P, Lemmens G, Robberecht W. Minocycline delays disease
onset and mortality in a transgenic model of ALS. NeuroReport 2002; 13(8):
1067-1070.
http://dx.doi.org/10.1097/00001756-200206120-00018
56.
Zhu S et al. Minocycline inhibits cytochrome c release and delays progression
of amyotrophic lateral sclerosis in mice. Nature 2002; 417: 74–78.
http://dx.doi.org/10.1038/417074a
57.
Gordon PH, Moore DH, Gelinas DF, Qualls C, Meister ME, Werner J, Mendoza M,
Mass J,Kushner G, Miller RG. Placebo-controlled phase I/II studies of
minocycline in amyotrophic lateral sclerosis. Neurology 2004; 62: 1845–1847.
http://dx.doi.org/10.1212/01.WNL.0000125321.92112.7E
58.
Pontieri FE, Ricci A, Pellicano C, Benincasa D, Buttarelli FR. Minocycline in
amyotrophic lateral sclerosis: a pilot study. Neurol Sci 2005; 26: 285–287.
http://dx.doi.org/10.1007/s10072-005-0474-x
59.
Gordon PH et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis:
a phase III randomised trial. Lancet Neurol 2007; 6: 1045–1053.
http://dx.doi.org/10.1016/S1474-4422(07)70270-3
60.
Shi P, Gal J, Kwinter D, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic
lateral sclerosis. Biochim Biophys Acta 2010; 1802(1): 45-51.
http://dx.doi.org/10.1016/j.bbadis.2009.08.012
61.
Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G.
Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic
lateral sclerosis induces mitochondrial alteration and increase of cytosolic
Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett 1997;
414: 365–368.
http://dx.doi.org/10.1016/S0014-5793(97)01051-X
62.
Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation
increases vulnerability of motor neurons to excitotoxicity by a mechanism
involving increased oxidative stress and perturbed calcium homeostasis. Exp
Neurol 1999; 160: 28–39.
http://dx.doi.org/10.1006/exnr.1999.7190
63.
Sasaki S, Iwata M. Ultrastructural study of synapses in the anterior horn
neurons of patients with amyotrophic lateral sclerosis. Neurosci Lett 1996;
204: 53–56.
http://dx.doi.org/10.1016/0304-3940(96)12314-4
64.
Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol
Cell Biochem 1994; 133/134: 193-220.
http://dx.doi.org/10.1007/BF01267955
65.
Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M,
Wokke JHJ, Franssen H, van den Berg LH. A randomized sequential trial of
creatine in amyotrophic lateral sclerosis. Ann Neurol 2003; 53:437-445.
http://dx.doi.org/10.1002/ana.10554
66.
Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine
against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J
Neurochem 2000; 74(5):1968–1978.
http://dx.doi.org/10.1046/j.1471-4159.2000.0741968.x
67.
Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of
creatine. Biochem Biophys Res Commun 2002; 290: 47–52.
http://dx.doi.org/10.1006/bbrc.2001.6164
68.
Wendt S, Dedeoglu A, Speer O, Wallimann T, Beal MF, Andreassen OA. Reduced
creatine kinase activity in transgenic amyotrophic lateral sclerosis mice. Free
Radic Biol Med 2002; 32(9): 920–926.
69.
Dolder M, Wendt S, Wallimann T. Mitochondrial creatine kinase in contact sites:
interaction with porin and adenine nucleotide translocase, role in permeability
transition and sensitivity to oxidative damage. Biol Signals Recept 2001; 10:
93–111.
http://dx.doi.org/10.1159/000046878
70.
Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA,
Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of
creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature
Med 1999; 5(3): 347-350.
http://dx.doi.org/10.1038/6568
71.
Snow RJ, Turnbull J, Silva DS, Jiang F. Creatine supplementation and riluzole
treatment provide similar beneficial effects in copper, zinc superoxide
dismutase (G93A) transgenic mice. Neurosci 2003; 119: 661-667.
http://dx.doi.org/10.1016/S0306-4522(03)00212-4
72.
Zhang W, Narayanan M, Friedlander RM. Additive neuroprotective effects of
minocycline with creatine in a mouse model of ALS. Ann Neurol 2003; 53:267-270.
http://dx.doi.org/10.1002/ana.10476
73.
Shefner JM et al. A clinical trial of creatine in ALS. Neurol 2004; 63:
1656–1661.
http://dx.doi.org/10.1212/01.WNL.0000142992.81995.F0
74.
Rosenfeld J, King RM, Jackson CE, Bedlack RS, Barohn RJ, Dick A, Phillips LH,
Chapin J, Gelinas DF, Lou J-S. Creatine monohydrate in ALS: Effects on
strength, fatigue, respiratory status and ALSFRS. Amyotroph Lateral Sc 2008; 9:
266-272.
http://dx.doi.org/10.1080/17482960802028890
75.
Ravits J et al. Deciphering amyotrophic lateral sclerosis: what phenotype,
neuropathology and genetics are telling us about pathogenesis. Amyotroph
Lateral Scler Frontotemporal Degener 2013; 14(Suppl 1): 5–18.
http://dx.doi.org/10.3109/21678421.2013.778548
76.
Beghi E et al. The epidemiology and treatment of ALS: Focus on the
heterogeneity of the disease and critical appraisal of therapeutic trials.
Amyotrophic Lateral Sclerosis 2011; 12: 1–10.
http://dx.doi.org/10.3109/17482968.2010.502940
77.
Hackam D, Redelmeier D. Translation of Research Evidence From Animals to
Humans. J Am Med Assoc 2006; 296(14): 1731-1732.
http://dx.doi.org/10.1001/jama.296.14.1731
78.
Seok J et al. Genomic responses in mouse models poorly mimic human inflammatory
diseases. Proc Natl Acad Sci 2013; 110(9): 3507–3512.
http://dx.doi.org/10.1073/pnas.1222878110
79.
Scott S et al. Design, power, and interpretation of studies in the standard
murine model of ALS. Amyotroph Lateral Sc 2008; 9: 4–15.
http://dx.doi.org/10.1080/17482960701856300
80.
Dal Canto MC, Gurney ME. A low expressor line of transgenic mice carrying a
mutant human Cu,Zn superoxide dismutase (SOD1) gene developes pathological
changes that most closely resemble those in human amyotrophic lateral
sclerosis. Acta Neuropathol 1997; 93(6): 537-550.
http://dx.doi.org/10.1007/s004010050650
81.
Saeed M, Yang Y, Deng H-X, Hung W-Y, Siddique N, Dellafave L, Gellera C,
Andersen PM, Siddique T. Age and founder effect of SOD1 A4V mutation causing ALS.
Neurol 2009; 72: 1634-1639.
http://dx.doi.org/10.1212/01.wnl.0000343509.76828.2a
82.
Deng H-X et al. Conversion to the amyotrophic lateral sclerosis phenotype is
associated with intermolecular linked insoluble aggregates of SOD1 in
mitochondria. Proc Natl Acad Sci 2006; 103(18): 7142-7147.
83.
Zwiegers P, Lee G, Shaw CA. Reduction in hSOD1 copy number significantly
impacts ALS phenotype presentation in G37R (line 29) mice: implications for the
assessment of putative therapeutic agents. J Neg Resul Biomed 2014; 13: 14.
84.
Alexander G, Erwin K, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP,
Heiman-Patterson TD. Effect of transgene copy number on survival in the G93A
SOD1 transgenic mouse model of ALS. J Mol Brain Res 2004; 130: 7–15.
http://dx.doi.org/10.1016/j.molbrainres.2004.07.002
PMid:15519671.
85.
Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME.
Age-dependent penetrance of disease in a transgenic mouse model of familial
amyotrophic lateral sclerosis. Mol Cell Neurosci 1995; 6: 349–362.
http://dx.doi.org/10.1006/mcne.1995.1027
86.
Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM-Y. Neurofilaments and
orthograde transport are reduced in ventral root axons of transgenic mice that
express human SOD1 with a G93A mutation. J Cell Biol 1997; 139(5): 1307–1315.
http://dx.doi.org/10.1083/jcb.139.5.1307
87.
Kalaria RN, Mitchell MJ, Harik SI. Correlation of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity with blood-brain
barrier monoamine oxidase activity. Proc Natl Acad Sci s 1987; 84: 3521–3525.
88.
Robelin L, Gonzalez De Aguilar JL. Blood Biomarkers for Amyotrophic Lateral
Sclerosis: Myth or Reality? BioMed Res Intl 2014; 2014.
89.
Ryberg H, Bowser R. Protein biomarkers for amyotrophic lateral sclerosis.
Expert Rev Proteomics 2008; 5(2): 249-262.
http://dx.doi.org/10.1586/14789450.5.2.249
90.
Macchi Z et al. A multi-center screening trial of rasagiline in patients with
amyotrophic lateral sclerosis: Possible mitochondrial biomarker target
engagement. Amyotroph Lateral Scler Frontotemporal Degener 2015: 1–8.
http://dx.doi.org/10.3109/21678421.2015.1026826
91.
Tabata RC, Wilson JMB, Ly P, Zwiegers P, Kwok D, Van Kampen JM, Cashman N, Shaw
CA. Chronic Exposure to Dietary Sterol Glucosides is Neurotoxic to Motor
Neurons and Induces an ALS–PDC Phenotype. Neuromol Med 2008; 10: 24–39.
http://dx.doi.org/10.1007/s12017-007-8020-z
92.
Schulz JD, Wilson J, Shaw CA. A Murine Model of ALS PDC with Behavioral and
Neuropathological Features of Parkinsonism. Ann New York Acad Sci 2003; 991:
326–329.
http://dx.doi.org/10.1111/j.1749-6632.2003.tb07497.x
93.
Wilson JMB et al. Behavioral and neurological correlates of ALS-parkinsonism
dementia complex in adult mice fed washed cycad flour. Neuromol Med 2002; 207-221.
94.
Kihira T, Yoshida S, Kondo T, Yase Y, Ono S. ALS-like skin changes in mice on a
chronic low-Ca/Mg high-Al diet. J Neurol Sci 2004; 219: 7-14.
http://dx.doi.org/10.1016/j.jns.2003.11.010
95.
Wegorzewska I, Bell S, Cairns NJ. TDP-43 mutant transgenic mice develop
features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci 2009;
106(44):18809-18814.
http://dx.doi.org/10.1073/pnas.0908767106
96.
Hukema R, Riemslagh F, Melhem S, Linde H, Severijnen L-A, Edbauer D, Maas A,
Charlet-Berguerand N, Willemsen R, Swieten J. A new inducible transgenic mouse
model for C9orf72-associated GGGGCC repeat expansion supports a
gain-of-function mechanism in C9orf72-associated ALS and FTD. Acta Neuropathol
Commun 2014; 2: 166.
http://dx.doi.org/10.1186/s40478-014-0166-y
97.
Chew J et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43
pathology, neuronal loss, and behavioral deficits. Science 2015; 348(6239):
1151–4.
http://dx.doi.org/10.1126/science.aaa9344
98.
Burkhardt MF et al. A cellular model for sporadic ALS using patient-derived
induced pluripotent stem cells. Mol Cell Neurosci 2013; 56: 355–64.
http://dx.doi.org/10.1016/j.mcn.2013.07.007
99.
Richard J-P, Maragakis NJ. Induced pluripotent stem cells from ALS patients for
disease modeling. Brain Res 2015; 1607: 15–25.
http://dx.doi.org/10.1016/j.brainres.2014.09.017
100.
Vaccaro A, Patten SA, Ciura S, Maios C, Therrien M, Drapeau P, Kabashi E,
Parker JA. Methylene blue protects against TDP-43 and FUS neuronal toxicity in
C. elegans and D. rerio. PLoS ONE 2012; 7: e42117.
http://dx.doi.org/10.1371/journal.pone.0042117
101.
Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis.
Nat Rev Neurosci 2013; 14(4): 248-264.
http://dx.doi.org/10.1038/nrn3430
102.
Ezzi S, Lariviere R, Urushitani M, Julien J-P. Neuronal over-expression of
chromogranin A accelerates disease onset in a mouse model of ALS. J Neurochem
2010; 115: 1102–1111.
http://dx.doi.org/10.1111/j.1471-4159.2010.06979.x
103.
Nguyen M, Lariviere R, Julien J-P. Deregulation of Cdk5 in a Mouse Model of
ALS: Toxicity Alleviated by Perikaryal Neurofilament Inclusions. Neuron 2001;
30:135-147.